The influence of Akkermansia muciniphila on intestinal barrier function
- PMID: 39097746
- PMCID: PMC11297771
- DOI: 10.1186/s13099-024-00635-7
The influence of Akkermansia muciniphila on intestinal barrier function
Abstract
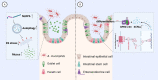
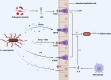
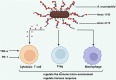
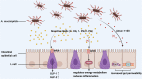
Intestinal barriers play a crucial role in human physiology, both in homeostatic and pathological conditions. Disruption of the intestinal barrier is a significant factor in the pathogenesis of gastrointestinal inflammatory diseases, such as inflammatory bowel disease. The profound influence of the gut microbiota on intestinal diseases has sparked considerable interest in manipulating it through dietary interventions, probiotics, and fecal microbiota transplantation as potential approaches to enhance the integrity of the intestinal barrier. Numerous studies have underscored the protective effects of specific microbiota and their associated metabolites. In recent years, an increasing body of research has demonstrated that Akkermansia muciniphila (A. muciniphila, Am) plays a beneficial role in various diseases, including diabetes, obesity, aging, cancer, and metabolic syndrome. It is gaining popularity as a regulator that influences the intestinal flora and intestinal barrier and is recognized as a 'new generation of probiotics'. Consequently, it may represent a potential target and promising therapy option for intestinal diseases. This article systematically summarizes the role of Am in the gut. Specifically, we carefully discuss key scientific issues that need resolution in the future regarding beneficial bacteria represented by Am, which may provide insights for the application of drugs targeting Am in clinical treatment.
Keywords: Akkermansia muciniphila; Cross-feeding; Immunity; Inflammation; Intestinal barrier.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

