Iron Overloading Potentiates the Antitumor Activity of 5-Fluorouracil by Promoting Apoptosis and Ferroptosis in Colorectal Cancer Cells
- PMID: 39097854
- PMCID: PMC11576816
- DOI: 10.1007/s12013-024-01463-x
Iron Overloading Potentiates the Antitumor Activity of 5-Fluorouracil by Promoting Apoptosis and Ferroptosis in Colorectal Cancer Cells
Abstract
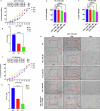
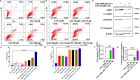
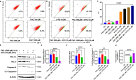
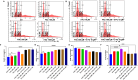
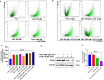
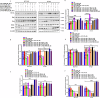
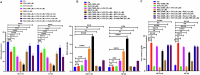
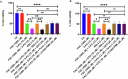
Resistance to 5-fluorouracil (5-FU) remains a significant challenge in colorectal cancer (CRC) treatment. Ferric ammonium citrate (FAC) is commonly used as an iron supplement due to its food-fortification properties; however, its potential role as a chemosensitizer in cancer therapy has not been studied. In this study, we explored the ability of FAC to sensitize CRC cells and increase their susceptibility to 5-FU-mediated anticancer effects. We assessed cell viability, cell cycle progression, apoptosis, mitochondrial membrane potential (MMP), reactive oxygen species (ROS) levels, ferroptosis, and iron metabolism-related protein expression using two CRC cell lines. Additionally, we conducted in silico analyses to compare iron markers in normal colon and CRC tumor tissues. Compared to controls, CRC cells pretreated with FAC and then treated with 5-FU exhibited significantly reduced growth and viability, along with increased ROS-mediated ferroptosis. Mechanistically, FAC-pretreated then 5-FU-treated CRC cells showed enhanced apoptosis, increased Bak/Bax expression, MMP depolarization, and decreased antiapoptotic protein levels (Bcl-2 and Bcl-xL). This combined treatment also led to G2/M cell cycle arrest, upregulation of p21 and p27, and downregulation of cyclin D1, c-Myc, survivin, and GPX4. Analysis of human colon tumor tissue revealed decreased expression of IRP-1, HMOX-1, and FTH1 but increased HAMP expression. In contrast, FAC-pretreated/5-FU-treated CRC cells exhibited a reverse pattern, suggesting that FAC-induced chemosensitization enhances 5-FU-mediated anticancer activity in CRC by disrupting iron homeostasis. These findings highlight the potential of iron overload as a chemosensitization strategy for improving CRC chemotherapy.
Keywords: Apoptosis; Chemosensitivity; Colorectal cancer; Ferric ammonium citrate; Ferroptosis; Iron overload.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Narayan, S., Raza, A., Mahmud, I., Koo, N., Garrett, T. J., Law, M. E., Law, B. K., & Sharma, A. K. (2022). Sensitization of FOLFOX-resistant colorectal cancer cells via the modulation of a novel pathway involving protein phosphatase 2A. Iscience, 25, 104518. 10.1016/j.isci.2022.104518. - PMC - PubMed
-
- Xue, X., Ramakrishnan, S. K., Weisz, K., Triner, D., Xie, L., Attili, D., Pant, A., Győrffy, B., Zhan, M., & Carter-Su, C. (2016). Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metabolism, 24, 447–461. 10.1016/j.cmet.2016.07.015. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

