Deciphering the neuroprotective mechanisms of RACK1 in cerebral ischemia-reperfusion injury: Pioneering insights into mitochondrial autophagy and the PINK1/Parkin axis
- PMID: 39097918
- PMCID: PMC11298203
- DOI: 10.1111/cns.14836
Deciphering the neuroprotective mechanisms of RACK1 in cerebral ischemia-reperfusion injury: Pioneering insights into mitochondrial autophagy and the PINK1/Parkin axis
Abstract
Introduction: Cerebral ischemia-reperfusion injury (CIRI) is a common and debilitating complication of cerebrovascular diseases such as stroke, characterized by mitochondrial dysfunction and cell apoptosis. Unraveling the molecular mechanisms behind these processes is essential for developing effective CIRI treatments. This study investigates the role of RACK1 (receptor for activated C kinase 1) in CIRI and its impact on mitochondrial autophagy.
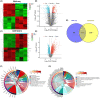
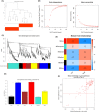
Methods: We utilized high-throughput transcriptome sequencing and weighted gene co-expression network analysis (WGCNA) to identify core genes associated with CIRI. In vitro experiments used human neuroblastoma SK-N-SH cells subjected to oxygen and glucose deprivation (OGD) to simulate ischemia, followed by reperfusion (OGD/R). RACK1 knockout cells were created using CRISPR/Cas9 technology, and cell viability, apoptosis, and mitochondrial function were assessed. In vivo experiments involved middle cerebral artery occlusion/reperfusion (MCAO/R) surgery in rats, evaluating neurological function and cell apoptosis.
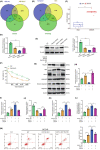
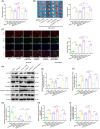
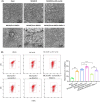
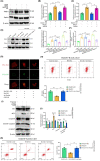
Results: Our findings revealed that RACK1 expression increases during CIRI and is protective by regulating mitochondrial autophagy through the PINK1/Parkin pathway. In vitro, RACK1 knockout exacerbated cell apoptosis, while overexpression of RACK1 reversed this process, enhancing mitochondrial function. In vivo, RACK1 overexpression reduced cerebral infarct volume and improved neurological deficits. The regulatory role of RACK1 depended on the PINK1/Parkin pathway, with RACK1 knockout inhibiting PINK1 and Parkin expression, while RACK1 overexpression restored them.
Conclusion: This study demonstrates that RACK1 safeguards against neural damage in CIRI by promoting mitochondrial autophagy through the PINK1/Parkin pathway. These findings offer crucial insights into the regulation of mitochondrial autophagy and cell apoptosis by RACK1, providing a promising foundation for future CIRI treatments.
Keywords: PINK/Parkin pathway; RACK1; cell apoptosis; cerebral ischemia‐reperfusion injury; mitochondrial autophagy; mitochondrial function; neuroprotection.
© 2024 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

