Stroke Research Using Administrative Claims Database in Japan: A Narrative Review
- PMID: 39098041
- PMCID: PMC11456354
- DOI: 10.5551/jat.RV22022
Stroke Research Using Administrative Claims Database in Japan: A Narrative Review
Abstract
Aims: Although administrative claims databases have recently been used for clinical research in Japan, no detailed description of their utilization in stroke research is available. We reviewed stroke studies using the Diagnosis Procedure Combination (DPC), the National Database of Health Insurance Claims and Specific Health Checkups (NDB), and several commercial databases sourced from social health insurance associations, focusing on their applications and limitations.
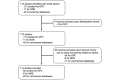
Methods: Original articles on stroke published by April 2024 using the DPC, NDB, and commercial databases were identified in Ovid MEDLINE. The characteristics of each database were compared in terms of comprehensiveness, traceability, baseline information, and outcome assessment in stroke research.
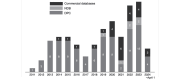
Results: A total of 114 studies were included (83 for DPC, 6 for NDB, and 25 for commercial databases). The number of stroke studies using administrative databases in Japan is still approximately 10 per year, although there is a slowly increasing trend. The DPC database was utilized for short-term outcome studies because of its detailed baseline and outcome information, although the inability to track patients once they changed facilities limits their use in long-term studies. The NDB database is potentially useful for long-term studies because of its comprehensiveness and traceability, but difficulties in data access restrict its usage. The most commonly used commercial database utilizes baseline information on lifestyle and blood test data, although the lack of coverage for those over 75 years old may limit its generalizability.
Conclusions: Administrative claims databases are beginning to be used in stroke research in Japan but are not yet fully utilized. Researchers need to understand their applications and limitations.
Keywords: Administrative claims database; Diagnosis Procedure Combination (DPC) database; National Database of Health Insurance Claims; Specific Health Checkups (NDB) database; Stroke.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
Similar articles
-
A Review of Studies Using Japanese Nationwide Administrative Claims Databases.Ann Clin Epidemiol. 2023 Jan 28;5(2):58-64. doi: 10.37737/ace.23008. eCollection 2023. Ann Clin Epidemiol. 2023. PMID: 38505730 Free PMC article. Review.
-
A Review of Research Studies Using Data from the Administrative Claims Databases in Japan.Drugs Real World Outcomes. 2022 Dec;9(4):543-550. doi: 10.1007/s40801-022-00331-5. Epub 2022 Sep 15. Drugs Real World Outcomes. 2022. PMID: 36107390 Free PMC article. Review.
-
Clinical research using real-world data: A narrative review.Respir Investig. 2024 Nov;62(6):929-934. doi: 10.1016/j.resinv.2024.08.002. Epub 2024 Aug 24. Respir Investig. 2024. PMID: 39182397 Review.
-
Updated Information on NDB.Ann Clin Epidemiol. 2024 Jun 13;6(3):73-76. doi: 10.37737/ace.24011. eCollection 2024. Ann Clin Epidemiol. 2024. PMID: 39034945 Free PMC article.
-
Literature Review of Studies Using the National Database of the Health Insurance Claims of Japan (NDB): Limitations and Strategies in Using the NDB for Research.JMA J. 2024 Jan 15;7(1):10-20. doi: 10.31662/jmaj.2023-0078. Epub 2023 Dec 27. JMA J. 2024. PMID: 38314426 Free PMC article. Review.
References
-
- Suissa S, and Garbe E: Administrative health databases in observational studies of drug effects--advantages and disadvantages. Nat Clin Pract Rheumatol, 2007; 3: 725-732 - PubMed
-
- Ministry of Health, Labour and Welfare, Japan: Ministerial Order on Standards for the Conduct of Post-Marketing Surveillance and Testing of Pharmaceuticals [Internet]: [cited 2024 April 1]. Available from: https: //www.mhlw.go.jp/web/t_doc?dataId=81aa6623&dataType=0&pageNo=1
-
- Harpe SE: Using secondary data sources for pharmacoepidemiology and outcomes research. Pharmacotherapy, 2009; 29: 138-153 - PubMed
-
- Japanese Society of Pharmacoepidemiology: Survey of applicable databases for clinical and pharmacoepidemiology in Japan. [Internet]: [cited 2024 April 1]. Available from: https: //sites.google.com/view/jspe-database-ja/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

