Nicotine inhalation and metabolism triggers AOX-mediated superoxide generation with oxidative lung injury
- PMID: 39098528
- PMCID: PMC11403528
- DOI: 10.1016/j.jbc.2024.107626
Nicotine inhalation and metabolism triggers AOX-mediated superoxide generation with oxidative lung injury
Abstract
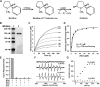
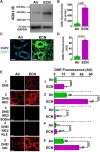
With the increasing use of vaping devices that deliver high levels of nicotine (NIC) to the lungs, sporadic lung injury has been observed. Commercial vaping solutions can contain high NIC concentrations of 150 mM or more. With high NIC levels, its metabolic products may induce toxicity. NIC is primarily metabolized to form NIC iminium (NICI) which is further metabolized by aldehyde oxidase (AOX) to cotinine. We determine that NICI in the presence of AOX is a potent trigger of superoxide generation. NICI stimulated superoxide generation from AOX with Km = 2.7 μM and Vmax = 794 nmol/min/mg measured by cytochrome-c reduction. EPR spin-trapping confirmed that NICI in the presence of AOX is a potent source of superoxide. AOX is expressed in the lungs and chronic e-cigarette exposure in mice greatly increased AOX expression. NICI or NIC stimulated superoxide production in the lungs of control mice with an even greater increase after chronic e-cigarette exposure. This superoxide production was quenched by AOX inhibition. Furthermore, e-cigarette-mediated NIC delivery triggered oxidative lung damage that was blocked by AOX inhibition. Thus, NIC metabolism triggers AOX-mediated superoxide generation that can cause lung injury. Therefore, high uncontrolled levels of NIC inhalation, as occur with e-cigarette use, can induce oxidative lung damage.
Keywords: EPR spin-trapping; aldehyde oxidase; electronic cigarettes; free radicals; superoxide.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

