Discovering novel plasma biomarkers for ischemic stroke: Lipidomic and metabolomic analyses in an aged mouse model
- PMID: 39098585
- PMCID: PMC11399596
- DOI: 10.1016/j.jlr.2024.100614
Discovering novel plasma biomarkers for ischemic stroke: Lipidomic and metabolomic analyses in an aged mouse model
Abstract
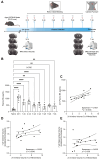
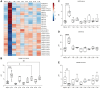
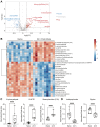
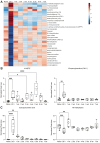
Ischemic stroke remains a leading cause of mortality and long-term disability worldwide, necessitating efforts to identify biomarkers for diagnosis, prognosis, and treatment monitoring. The present study aimed to identify novel plasma biomarkers of neurodegeneration and inflammation in a mouse model of stroke induced by distal middle cerebral artery occlusion. Using targeted lipidomic and global untargeted metabolomic profiling of plasma collected from aged male mice 24 h after stroke and weekly thereafter for 7 weeks, we discovered distinct acute and chronic signatures. In the acute phase, we observed elevations in myelin-associated lipids, including sphingomyelin (SM) and hexosylceramide (HCER) lipid species, indicating brain lipid catabolism. In the chronic phase, we identified 12-hydroxyeicosatetraenoic acid (12-HETE) as a putative biomarker of prolonged inflammation, consistent with our previous observation of a biphasic pro-inflammatory response to ischemia in the mouse brain. These results provide insight into the metabolic alterations detectable in the plasma after stroke and highlight the potential of myelin degradation products and arachidonic acid derivatives as biomarkers of neurodegeneration and inflammation, respectively. These discoveries lay the groundwork for further validation in human studies and may improve stroke management strategies.
Keywords: 12-HETE; arachidonic acid; brain lipids; inflammation; lipids; lipoxygenase; myelin; neurodegeneration; neurofilament light; sphingolipids.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Lindsay M.P., Norrving B., Sacco R.L., Brainin M., Hacke W., Martins S., et al. World stroke organization (WSO): global stroke fact sheet 2019. Int. J. Stroke. 2019;14:806–817. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

