KCTD17-mediated Ras stabilization promotes hepatocellular carcinoma progression
- PMID: 39098817
- PMCID: PMC11540369
- DOI: 10.3350/cmh.2024.0364
KCTD17-mediated Ras stabilization promotes hepatocellular carcinoma progression
Abstract
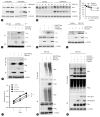
Background/aims: Potassium channel tetramerization domain containing 17 (KCTD17) protein, an adaptor for the cullin3 (Cul3) ubiquitin ligase complex, has been implicated in various human diseases; however, its role in hepatocellular carcinoma (HCC) remains elusive. Here, we aimed to elucidate the clinical features of KCTD17, and investigate the mechanisms by which KCTD17 affects HCC progression.
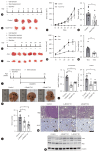
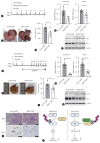
Methods: We analyzed transcriptomic data from patients with HCC. Hepatocyte-specific KCTD17 deficient mice were treated with diethylnitrosamine (DEN) to assess its effect on HCC progression. Additionally, we tested KCTD17-directed antisense oligonucleotides for their therapeutic potential in vivo.
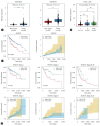
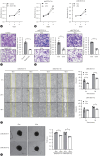
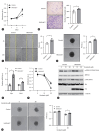
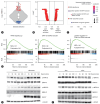
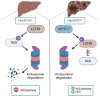
Results: Our investigation revealed the upregulation of KCTD17 expression in both tumors from patients with HCC and mouse models of HCC, in comparison to non-tumor controls. We identified the leucine zipper-like transcriptional regulator 1 (Lztr1) protein, a previously identified Ras destabilizer, as a substrate for KCTD17-Cul3 complex. KCTD17-mediated Lztr1 degradation led to Ras stabilization, resulting in increased proliferation, migration, and wound healing in liver cancer cells. Hepatocyte-specific KCTD17 deficient mice or liver cancer xenograft models were less susceptible to carcinogenesis or tumor growth. Similarly, treatment with KCTD17-directed antisense oligonucleotides (ASO) in a mouse model of HCC markedly lowered tumor volume as well as Ras protein levels, compared to those in control ASO-treated mice.
Conclusion: KCTD17 induces the stabilization of Ras and downstream signaling pathways and HCC progression and may represent a novel therapeutic target for HCC.
Keywords: Antisense oligonucleotides; HCC; KCTD17; Lztr1; Ras.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures
References
-
- Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, et al. EUROCARE-5 Working Group Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999-2007: results of EUROCARE-5. Eur J Cancer. 2015;51:2169–2178. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Zhu AX, Kudo M, Assenat E, Cattan S, Kang YK, Lim HY, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312:57–67. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

