Prebiotic thiol-catalyzed thioamide bond formation
- PMID: 39098875
- PMCID: PMC11299287
- DOI: 10.1186/s12932-024-00088-6
Prebiotic thiol-catalyzed thioamide bond formation
Abstract
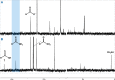
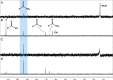
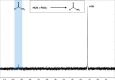
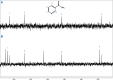
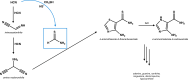
Thioamide bonds are important intermediates in prebiotic chemistry. In cyanosulfidic prebiotic chemistry, they serve as crucial intermediates in the pathways that lead to the formation of many important biomolecules (e.g., amino acids). They can also serve as purine and pyrimidine precursors, the two classes of heterocycle employed in genetic molecules. Despite their importance, the formation of thioamide bonds from nitriles under prebiotic conditions has required large excesses of sulfide or compounds with unknown prebiotic sources. Here, we describe the thiol-catalyzed formation of thioamide bonds from nitriles. We show that the formation of the simplest of these compounds, thioformamide, forms readily in spark-discharge experiments from hydrogen cyanide, sulfide, and a methanethiol catalyst, suggesting potential accumulation on early Earth. Lastly, we demonstrate that thioformamide has a Gibbs energy of hydrolysis ( ) comparable to other energy-currencies on early Earth such as pyrophosphate and thioester bonds. Overall, our findings imply that thioamides might have been abundant on early Earth and served a variety of functions during chemical evolution.
Keywords: Hydrogen cyanide; Origin of life; Proto-metabolism; Thioformamide.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no Conflict of interest.
Figures

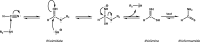


Similar articles
-
Thioesters provide a plausible prebiotic path to proto-peptides.Nat Commun. 2022 May 11;13(1):2569. doi: 10.1038/s41467-022-30191-0. Nat Commun. 2022. PMID: 35562173 Free PMC article.
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
-
Prebiotic chemical refugia: multifaceted scenario for the formation of biomolecules in primitive Earth.Theory Biosci. 2022 Nov;141(4):339-347. doi: 10.1007/s12064-022-00377-7. Epub 2022 Aug 30. Theory Biosci. 2022. PMID: 36042123 Review.
-
Characterization of the Simplest Thiolimine: The Higher Energy Tautomer of Thioformamide.Chemistry. 2021 Apr 16;27(22):6732-6739. doi: 10.1002/chem.202005188. Epub 2021 Mar 12. Chemistry. 2021. PMID: 33496350 Free PMC article.
-
Prebiotic syntheses of purines and pyrimidines.Adv Space Res. 1984;4(12):125-31. doi: 10.1016/0273-1177(84)90554-4. Adv Space Res. 1984. PMID: 11537766 Review.
Cited by
-
Rapid hydrolysis rates of thio- and phosphate esters constrain the origin of metabolism to cool, acidic to neutral environments.iScience. 2024 Oct 3;27(11):111088. doi: 10.1016/j.isci.2024.111088. eCollection 2024 Nov 15. iScience. 2024. PMID: 39493872 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

