A region-confined PROTAC nanoplatform for spatiotemporally tunable protein degradation and enhanced cancer therapy
- PMID: 39098906
- PMCID: PMC11298519
- DOI: 10.1038/s41467-024-50735-w
A region-confined PROTAC nanoplatform for spatiotemporally tunable protein degradation and enhanced cancer therapy
Abstract
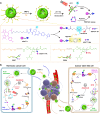
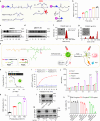
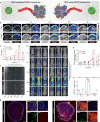
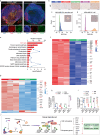
The antitumor performance of PROteolysis-TArgeting Chimeras (PROTACs) is limited by its insufficient tumor specificity and poor pharmacokinetics. These disadvantages are further compounded by tumor heterogeneity, especially the presence of cancer stem-like cells, which drive tumor growth and relapse. Herein, we design a region-confined PROTAC nanoplatform that integrates both reactive oxygen species (ROS)-activatable and hypoxia-responsive PROTAC prodrugs for the precise manipulation of bromodomain and extraterminal protein 4 expression and tumor eradication. These PROTAC nanoparticles selectively accumulate within and penetrate deep into tumors via response to matrix metalloproteinase-2. Photoactivity is then reactivated in response to the acidic intracellular milieu and the PROTAC is discharged due to the ROS generated via photodynamic therapy specifically within the normoxic microenvironment. Moreover, the latent hypoxia-responsive PROTAC prodrug is restored in hypoxic cancer stem-like cells overexpressing nitroreductase. Here, we show the ability of region-confined PROTAC nanoplatform to effectively degrade BRD4 in both normoxic and hypoxic environments, markedly hindering tumor progression in breast and head-neck tumor models.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- U22A20328/National Natural Science Foundation of China (National Science Foundation of China)
- 22074043/National Natural Science Foundation of China (National Science Foundation of China)
- 23ZR1475000/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 20430711800/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 19DZ2251100/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
LinkOut - more resources
Full Text Sources

