Targeting Lactobacillus johnsonii to reverse chronic kidney disease
- PMID: 39098923
- PMCID: PMC11298530
- DOI: 10.1038/s41392-024-01913-1
Targeting Lactobacillus johnsonii to reverse chronic kidney disease
Abstract
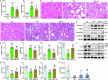
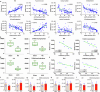
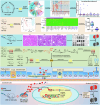
Accumulated evidence suggested that gut microbial dysbiosis interplayed with progressive chronic kidney disease (CKD). However, no available therapy is effective in suppressing progressive CKD. Here, using microbiomics in 480 participants including healthy controls and patients with stage 1-5 CKD, we identified an elongation taxonomic chain Bacilli-Lactobacillales-Lactobacillaceae-Lactobacillus-Lactobacillus johnsonii correlated with patients with CKD progression, whose abundance strongly correlated with clinical kidney markers. L. johnsonii abundance reduced with progressive CKD in rats with adenine-induced CKD. L. johnsonii supplementation ameliorated kidney lesion. Serum indole-3-aldehyde (IAld), whose level strongly negatively correlated with creatinine level in CKD rats, decreased in serum of rats induced using unilateral ureteral obstruction (UUO) and 5/6 nephrectomy (NX) as well as late CKD patients. Treatment with IAld dampened kidney lesion through suppressing aryl hydrocarbon receptor (AHR) signal in rats with CKD or UUO, and in cultured 1-hydroxypyrene-induced HK-2 cells. Renoprotective effect of IAld was partially diminished in AHR deficiency mice and HK-2 cells. Our further data showed that treatment with L. johnsonii attenuated kidney lesion by suppressing AHR signal via increasing serum IAld level. Taken together, targeting L. johnsonii might reverse patients with CKD. This study provides a deeper understanding of how microbial-produced tryptophan metabolism affects host disease and discovers potential pathways for prophylactic and therapeutic treatments for CKD patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
- 82074002/National Natural Science Foundation of China (National Science Foundation of China)
- 82274079/National Natural Science Foundation of China (National Science Foundation of China)
- 82274192/National Natural Science Foundation of China (National Science Foundation of China)
- 2023T160061/China Postdoctoral Science Foundation

