Safety and Immunogenicity of Respiratory Syncytial Virus Prefusion F Protein Vaccine when Co-administered with Adjuvanted Seasonal Quadrivalent Influenza Vaccine in Older Adults: A Phase 3 Randomized Trial
- PMID: 39099085
- PMCID: PMC11478588
- DOI: 10.1093/cid/ciae365
Safety and Immunogenicity of Respiratory Syncytial Virus Prefusion F Protein Vaccine when Co-administered with Adjuvanted Seasonal Quadrivalent Influenza Vaccine in Older Adults: A Phase 3 Randomized Trial
Abstract
Background: We evaluated co-administration of adjuvanted seasonal quadrivalent influenza vaccine (FLU-aQIV) and respiratory syncytial virus (RSV) prefusion F protein-based vaccine (RSVPreF3 OA) in ≥65-year-olds.
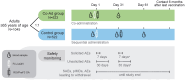
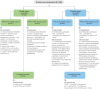
Methods: This phase 3, open-label trial randomized ≥65-year-olds to receive FLU-aQIV and RSVPreF3 OA concomitantly (Co-Ad) or sequentially, 1 month apart (Control). Primary objectives were to demonstrate the non-inferiority of FLU-aQIV and RSVPreF3 OA co-administration versus sequential administration in terms of hemagglutination inhibition (HI) titers for each FLU-aQIV strain and RSV-A and RSV-B neutralization titers, 1 month post-vaccination. Reactogenicity and safety were also assessed.
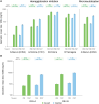
Results: Overall, 1045 participants were vaccinated (Co-Ad: 523; Control: 522). Non-inferiority of FLU-aQIV and RSVPreF3 OA co-administration versus sequential administration was demonstrated in terms of HI titers for the A/Victoria(H1N1), B/Victoria, and B/Yamagata influenza strains and RSV-A neutralization titers (upper limits [ULs] of 95% confidence intervals [CIs] for adjusted geometric mean titer [GMT] ratios [Control/Co-Ad] ≤1.50) but not for A/Darwin(H3N2) HI titers (95% CI UL = 1.53). The immune response to A/Darwin(H3N2) was further assessed post-hoc using a microneutralization assay; the post-vaccination adjusted GMT ratio (Control/Co-Ad) was 1.23 (95% CI: 1.06-1.42, ie, UL ≤1.50), suggesting an adequate immune response to A/Darwin(H3N2) following co-administration. RSV-B neutralization titers were comparable between groups (95% CI UL for adjusted GMT ratio ≤1.50). Solicited adverse events were mostly mild or moderate and transient; unsolicited and serious adverse event rates were balanced between groups.
Conclusions: Adjuvanted FLU-aQIV and RSVPreF3 OA had acceptable reactogenicity/safety profiles when co-administered in ≥65-year-olds, without clinically relevant interference with the immune responses to either vaccine.
Clinical trials registration: NCT05568797.
Keywords: RSVPreF3 OA; adjuvanted seasonal influenza vaccine; concurrent administration; immunogenicity; safety.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. R. C. runs RSV studies at her trial site with Moderna and GSK and received NIHR funding to attend the World Vaccine Congress (October 2022) as part of the UK delegation; she is the Deputy Specialty Lead and the Clinical Lead for vaccine research at the North West Coast Clinical Research Network. P. L. received consulting fees from GSK and Pfizer, declares payments or honoraria from Janssen, GSK, Moderna, AstraZeneca, Pfizer, and Sanofi, and financial support for attending meetings and/or travel from AstraZeneca, Pfizer, and Sanofi. J.-F. N., M. R., and I. S.-M. report grants and funding from GSK to their institutions for conducting this clinical trial. N. D., S. G., M.-P. D., A. J., H. A. H., S. K., and N. M. are or were employed by GSK at the time the study was designed, initiated, and/or conducted. N. D., M.-P. D., H. A. H., S. K., and N. M. have stock options or shares from GSK. N. D. also reports stocks from Haleon and patents on vaccination against RSV (numbers 2218080.6 and 2303002.6). M.-P. D. is a co-applicant on a pending patent filed by GSK. S. N. M. declares being a Principal Investigator on the current trial. S. D., J. L., H. M. M., M. P. V., and M. H. R. -R. have no conflicts of interest to declare. The authors declare no other financial or non-financial relationships. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


Comment in
-
We Have Effective Respiratory Syncytial Virus Vaccines to Prevent Disease in Adults: What Else Do We Need to Know About How to Use Them?Clin Infect Dis. 2024 Oct 15;79(4):1099-1101. doi: 10.1093/cid/ciae362. Clin Infect Dis. 2024. PMID: 39099081 No abstract available.
References
-
- Nam HH, Ison MG. Respiratory syncytial virus infection in adults. BMJ 2019; 366:l5021. - PubMed
-
- Shi T, Vennard S, Jasiewicz F, Brogden R, Nair H, RESCEU Investigators . Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis 2022; 226:S17–21. - PubMed
-
- Branche AR, Saiman L, Walsh EE, et al. . Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis 2022; 74:1004–11. - PubMed
-
- Prasad N, Walker TA, Waite B, et al. . Respiratory syncytial virus-associated hospitalizations among adults with chronic medical conditions. Clin Infect Dis 2021; 73:e158–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

