Noninferior Immunogenicity and Consistent Safety of Respiratory Syncytial Virus Prefusion F Protein Vaccine in Adults 50-59 Years Compared to ≥60 Years of Age
- PMID: 39099093
- PMCID: PMC11478578
- DOI: 10.1093/cid/ciae364
Noninferior Immunogenicity and Consistent Safety of Respiratory Syncytial Virus Prefusion F Protein Vaccine in Adults 50-59 Years Compared to ≥60 Years of Age
Abstract
Background: The adjuvanted respiratory syncytial virus (RSV) prefusion F protein-based vaccine (RSVPreF3 OA) is approved in adults aged ≥60 years. We evaluated RSVPreF3 OA immunogenicity and safety in adults aged 50-59 years without or with increased risk for RSV disease due to specific chronic medical conditions.
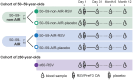
Methods: This observer-blind, phase 3, noninferiority trial included adults aged 50-59 years, stratified into 2 subcohorts: those with and those without predefined, stable, chronic medical conditions leading to an increased risk for RSV disease. Participants in both subcohorts were randomized 2:1 to receive RSVPreF3 OA or placebo. A control group of adults aged ≥60 years received RSVPreF3 OA. Primary outcomes were RSV-A and RSV-B neutralization titers (geometric mean titer ratios and sero-response rate differences) 1 month post-vaccination in 50-59-year-olds versus ≥60-year-olds. Cell-mediated immunity and safety were also assessed.
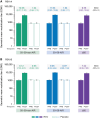
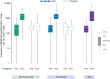
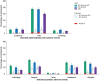
Results: The exposed population included 1152 participants aged 50-59 years and 381 participants aged ≥60 years. RSVPreF3 OA was immunologically noninferior in 50-59-year-olds versus ≥60-year-olds; noninferiority criteria were met for RSV-A and RSV-B neutralization titers in those with and those without increased risk for RSV disease. Frequencies of RSVPreF3-specific polyfunctional CD4+ T cells increased substantially from pre- to 1 month post-vaccination. Most solicited adverse events had mild-to-moderate intensity and were transient. Unsolicited and serious adverse event rates were similar in all groups.
Conclusions: RSVPreF3 OA was immunologically noninferior in 50-59-year-olds compared to ≥60-year-olds, in whom efficacy was previously demonstrated. The safety profile in 50-59-year-olds was consistent with that in ≥60-year-olds.
Clinical trial registration: ClinicalTrials.gov: NCT05590403.
Keywords: chronic conditions; humoral immunity; respiratory syncytial virus; safety; vaccination.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . H. A. H., M. d. H., B. S., S. D., M.-P. D., D. D., J. H., C. V., and V. H. are/were GSK employees at the time when the study was designed and/or conducted. H. A. H., M. d. H., B. S., S. D., M.-P. D., D. D., J. H., and V. H. hold shares in GSK as part of their employee remuneration. M.-P. D. is a co-applicant on a pending patent filed by GSK. M. F. received study-related payments for training and study conduct from GSK. T. F. S. received honoraria for lectures from AstraZeneca, Bavarian Nordic, Biogen, CSL-Seqirus, GSK, Janssen-Cilag, Merck-Serono, Moderna, Novavax, MSD, Pfizer, Roche, Sanofi-Aventis, and Takeda; he participated on advisory boards for Bavarian Nordic, CSL-Seqirus, BioNTech, GSK, Moderna, Novavax, and Takeda. S. A. N. declares study-related payments to his institution from GSK and support from GSK to attend investigators meetings. J. R.-G. declares study-related payments from GSK and honoraria and support for attending meetings and/or travel from GSK, Pfizer, and Sanofi-MSD; he also participated on data and safety monitoring boards or advisory boards from GSK and Pfizer. C. Z. received grants from GSK for the conduct of this study and support from GSK for attending meetings. J. G. declares study-related payments from GSK; grants from Novartis, Pharmalog, New Amsterdam Pharma, Syneos, Winecker Pharma, and Lilly; and consulting fees, honoraria, payment for expert testimony, and support for attending meetings and/or travel from GSK. C. V.-P. is a former employee of QPS Netherlands B.V. The other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


Comment in
-
We Have Effective Respiratory Syncytial Virus Vaccines to Prevent Disease in Adults: What Else Do We Need to Know About How to Use Them?Clin Infect Dis. 2024 Oct 15;79(4):1099-1101. doi: 10.1093/cid/ciae362. Clin Infect Dis. 2024. PMID: 39099081 No abstract available.
References
-
- Branche AR, Saiman L, Walsh EE, et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis 2022; 74:1004–11. - PubMed
-
- Prasad N, Walker TA, Waite B, et al. Respiratory syncytial virus-associated hospitalizations among adults with chronic medical conditions. Clin Infect Dis 2021; 73:e158–63. - PubMed
-
- Shi T, Vennard S, Jasiewicz F, Brogden R, Nair H; RESCEU Investigators . Disease burden estimates of respiratory syncytial virus related acute respiratory infections in adults with comorbidity: a systematic review and meta-analysis. J Infect Dis 2022; 226:S17–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

