Application of the international criteria for optic neuritis in the Acute Optic Neuritis Network
- PMID: 39099240
- PMCID: PMC11537134
- DOI: 10.1002/acn3.52166
Application of the international criteria for optic neuritis in the Acute Optic Neuritis Network
Abstract
Objective: The first international consensus criteria for optic neuritis (ICON) were published in 2022. We applied these criteria to a prospective, global observational study of acute optic neuritis (ON).
Methods: We included 160 patients with a first-ever acute ON suggestive of a demyelinating CNS disease from the Acute Optic Neuritis Network (ACON). We applied the 2022 ICON to all participants and subsequently adjusted the ICON by replacing a missing relative afferent pupillary defect (RAPD) or dyschromatopsia if magnetic resonance imaging pathology of the optical nerve plus optical coherence tomography abnormalities or certain biomarkers are present.
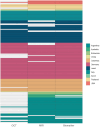
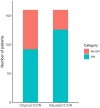
Results: According to the 2022 ICON, 80 (50%) patients were classified as definite ON, 12 (7%) patients were classified as possible ON, and 68 (43%) as not ON (NON). The main reasons for classification as NON were absent RAPD (52 patients, 76%) or dyschromatopsia (49 patients, 72%). Distribution of underlying ON etiologies was as follows: 78 (49%) patients had a single isolated ON, 41 (26%) patients were diagnosed with multiple sclerosis, 25 (16%) patients with myelin oligodendrocyte glycoprotein antibody-associated disease, and 15 (9%) with neuromyelitis optica spectrum disorder. The application of the adjusted ON criteria yielded a higher proportion of patients classified as ON (126 patients, 79%).
Interpretation: According to the 2022 ICON, almost half of the included patients in ACON did not fulfill the requirements for classification of definite or possible ON, particularly due to missing RAPD and dyschromatopsia. Thorough RAPD examination and formal color vision testing are critical to the application of the 2022 ICON.
© 2024 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
PK, RA, CB, OB, AB, SC, LC, YC‐T, JH, MH, NL, PL, IL, MBL, CM, AJMV, CO, SO, MO, JLPU, JS‐G, DS, NR, F‐DS, JS, IS, SS, AT, NT, IT, AVD, AW‐Y, TW, SZ, and LAZ did not report any disclosures. SA received speaker's honoraria from Bayer, Alexion, Roche, and research grants from Stiftung Charité, Fritz‐Thyssen‐Stiftung, HEAD Genuit Stiftung, Rahel Hirsch Program, Novartis, and Roche. JJC is a consultant to UCB and Horizon, unrelated to this study. ECC has received reimbursement for developing educational presentations, educational and research grants, consultation fees and/or travel stipends from Biogen Argentina y LATAM, Genzyme Argentina, Merck Argentina y LATAM, Roche Argentina y LATAM, Raffo, Novartis Argentina, MERZ Argentina, Biosidus, Astrazeneca Argentina, Horizon Global, Amgen Argentina, the Guthy‐Jackson Charitable Foundation (Los Angeles, CA, USA), The Sumaira Foundation (Boston, MA, USA) and LACTRIMS. RCD received research funding from the Star Scientific Foundation, The Trish Multiple Sclerosis Research Foundation, Multiple Sclerosis Research Australia, the Petre Foundation, and the NHMRC (Australia; Investigator Grant). He has also received honoraria from Biogen Idec as an invited speaker and is on the IDMC for a Roche RCT in pediatric MS. He is on the medical advisory board (nonremunerated position). EPF served on advisory boards for Alexion, Genentech, Horizon Therapeutics, and UCB. He has received research support from UCB. He has received speaker honoraria from Pharmacy Times. He received royalties from UpToDate. He is a site principal investigator in a randomized clinical trial of Rozanolixizumab for relapsing myelin oligodendrocyte glycoprotein antibody‐associated disease run by UCB. He is a site principal investigator and a member of the steering committee for a clinical trial of satralizumab for relapsing myelin oligodendrocyte glycoprotein antibody‐associated disease run by Roche/Genentech. He has received funding from the NIH (R01NS113828). Dr Flanagan is a member of the medical advisory board of the MOG project. He is an editorial board member of Neurology, Neuroimmunology and Neuroinflammation, The Journal of the Neurological Sciences and Neuroimmunology Reports. A patent has been submitted on DACH1‐IgG as a biomarker of paraneoplastic autoimmunity. JAG reports travel expenses and nonfinancial support from Merck, outside the submitted work. JH reports grants from Friedrich‐Baur‐Stiftung, Merck, and Horizon; personal fees and nonfinancial support from Alexion, Horizon, Roche, Merck, Novartis, Biogen, B.M.S., and Janssen; and nonfinancial support from the Guthy‐Jackson Charitable Foundation and The Sumaira Foundation. CH received research grants from Merck, Novartis, and speaker honoraria from Merck and Roche. SM received speakers' honoraria from Alexion, Novartis, Biogen, Sanofi, and Horizon unrelated to this study. FCO currently receives research funding from the Hertie Foundation for Excellence in Clinical Neurosciences and Novartis, both unrelated to this project. She also received fellowship support by the American Academy of Neurology (until 2023) and the National Multiple Sclerosis Society (until 2023), both unrelated to this project.
JS‐G serves as a co‐editor for Europe for the
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

