Comparison of Three Regional Medicines Regulatory Harmonisation Initiatives in Africa: Opportunities for Improvement and Alignment
- PMID: 39099506
- PMCID: PMC11270597
- DOI: 10.34172/ijhpm.2024.8070
Comparison of Three Regional Medicines Regulatory Harmonisation Initiatives in Africa: Opportunities for Improvement and Alignment
Abstract
Background: The African Medicines Regulatory Harmonisation (AMRH) Initiative was formed in 2009 and subsequently, three regional initiatives (East African Community Medicines Regulatory Harmonisation [MRH], Southern African Development Community [SADC]/ZaZiBoNa MRH, and the Economic Community of West Africa States MRH) were established. As these initiatives serve as a foundation for the African Medicines Agency (AMA), the aim of this study was to compare their operating models, successes and challenges to identify opportunities for improvement and alignment.
Methods: A mixed method approach was used for the data collection using a questionnaire, the Process, Effectiveness and Efficiency Rating (PEER), developed by the authors specifically for this study and semi-structured interview techniques. There were 23 study participants (one from each agency of the member countries of the three regions). It was hoped that data generated from this study would lead to a series of recommendations, which would then be ratified by the regulatory authorities.
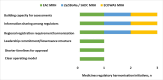
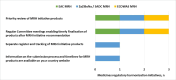
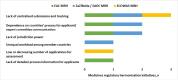
Results: Most respondents stated that AMRH contributed to the strengthening of regulatory systems and harmonising regulatory requirements across economic regions of Africa, potentially resulting in improved access to quality-assured medicines. Although established at different times and at the discretion of each region, the marketing authorisation application review processes are largely similar, with a few differences noted in the eligibility and submission requirements, type of procedures employed, the timelines and fees payable. The challenges identified in the three regions are also similar, with the most noteworthy being the lack of a binding legal framework for regional approvals.
Conclusion: In this study, we compared the process, successes and challenges of these three regional harmonisation initiatives in Africa addressing the areas of legal frameworks, information management systems, the accessibility and affordability of medicines and reliance that will bring greater alignment and efficiency in their operating models, thereby strengthening the foundation of the soon-to-be-operationalised AMA.
Keywords: African Medicines Agency; EAC; ECOWAS; Medicines Regulatory Harmonisation; SADC; ZaZiBoNa.
© 2024 The Author(s); Published by Kerman University of Medical Sciences This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Conflict of interest statement
Authors declare that they have no competing interests.
Figures
References
-
- Rägo L, Santoso B. Drug regulation: history, present and future. In: van Boxtel CJ, Santoso B, Edwards IR, eds. Drug Benefits and Risks: International Textbook of Clinical Pharmacology. 2nd ed. Uppsala: IOS Press, Uppsala Monitoring Centre; 2008.
-
- European Medicines Agency. EMA Starts First Rolling Review of a COVID-19 Vaccine in the EU. https://www.ema.europa.eu/en/news/ema-starts-first-rolling-review-covid-.... Accessed January 30, 2024.
-
- World Health Organization (WHO). TRS 1033 - Annex 10: Good Reliance Practices in the Regulation of Medical Products: High Level Principles and Considerations. WHO Expert Committee on Specifications for Pharmaceutical Preparations: Fifty-Fifth Report. WHO. 2021:237-267.
-
- World Health Organization (WHO). Essential medicines and health products. WHO Global Benchmarking Tool (GBT) for Evaluation of National Regulatory Systems. 2021. https://www.who.int/tools/global-benchmarking-tools/VI. Accessed January 30, 2024.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials