Exploring the effect of gut microbiome on Alzheimer's disease
- PMID: 39099604
- PMCID: PMC11296257
- DOI: 10.1016/j.bbrep.2024.101776
Exploring the effect of gut microbiome on Alzheimer's disease
Abstract
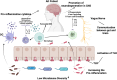
Alzheimer's disease (AD) is the most widespread and irreversible form of dementia and accounts for more than half of dementia cases. The most significant risk factors for AD are aging-related exacerbations, degradation of anatomical pathways, environmental variables and mitochondrial dysfunction. Finding a decisive therapeutic solution is a major current issue. Nuanced interactions between major neuropathological mechanisms in AD in patients and microbiome have recently gained rising attention. The presence of bacterial amyloid in the gut triggers the immune system, resulting in increased immune feedbacks and endogenous neuronal amyloid within the CNS. Also, early clinical research revealed that changing the microbiome with beneficial bacteria or probiotics could affect brain function in AD. New approaches focus on the possible neuroprotective action of disease-modifying medications in AD. In the present review, we discuss the impact of the gut microbiota on the brain and review emerging research that suggests a disruption in the microbiota-brain axis can affect AD by mediating neuroinflammation. Such novel methods could help the development of novel therapeutics for AD.
Keywords: Alzheimer's disease; Gut-brain axis; Microbiome; Neurodegenerative disorders.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Burns A., Iliffe S. Alzheimer's disease. BMJ. 2009;338 - PubMed
-
- Bennett D.A., Schneider J.A., Bienias J.L., Evans D.A., Wilson R.S. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64(5):834–841. - PubMed
-
- Cortés N., Andrade V., Maccioni R.B. Behavioral and neuropsychiatric disorders in Alzheimer's disease. J Alzheimers Dis. 2018;63(3):899–910. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

