11.7T Diffusion Magnetic Resonance Imaging and Tractography to Probe Human Brain Organoid Microstructure
- PMID: 39099731
- PMCID: PMC11295450
- DOI: 10.1016/j.bpsgos.2024.100344
11.7T Diffusion Magnetic Resonance Imaging and Tractography to Probe Human Brain Organoid Microstructure
Abstract
Background: Human brain organoids are 3-dimensional cellular models that mimic architectural features of a developing brain. Generated from human induced pluripotent stem cells, these organoids offer an unparalleled physiologically relevant in vitro system for disease modeling and drug screening. In the current study, we sought to establish a foundation for a magnetic resonance imaging (MRI)-based, label-free imaging system that offers high-resolution capabilities for deep tissue imaging of whole organoids.
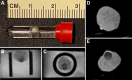
Methods: An 11.7T Bruker/89 mm microimaging system was used to collect high-resolution multishell 3-dimensional diffusion images of 2 induced pluripotent stem cell-derived human hippocampal brain organoids. The MRI features identified in the study were interpreted on the basis of similarities with immunofluorescence microscopy.
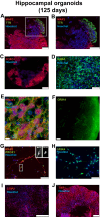
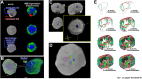
Results: MRI microscopy at ≤40 μm isotropic resolution provided a 3-dimensional view of organoid microstructure. T2-weighted contrast showed a rosette-like internal structure and a protruding spherical structure that correlated with immunofluorescence staining for the choroid plexus. Diffusion tractography methods can be used to model tissue microstructural features and possibly map neuronal organization. This approach complements traditional immunohistochemistry imaging methods without the need for tissue clearing.
Conclusions: This proof-of-concept study shows, for the first time, the application of high-resolution diffusion MRI microscopy to image 2-mm diameter spherical human brain organoids. Application of ultrahigh-field MRI and diffusion tractography is a powerful modality for whole organoid imaging and has the potential to make a significant impact for probing microstructural changes in brain organoids used to model psychiatric disorders, neurodegenerative diseases, and viral infections of the human brain, as well as for assessing neurotoxicity in drug screening.
Keywords: Differentiating rosette-like structures; Diffusion imaging microscopy; High-field MRI; Human brain organoids; Human induced pluripotent stem cells; Neuronal streamlines.
Plain language summary
Versace et al. present a groundbreaking approach using ultrahigh-resolution MRI (11.7T) for deep tissue imaging of whole human brain organoids. These 3D miniature brains mimic the developing brain’s architecture and hold promise for disease modeling and drug discovery. This label-free MRI approach offers the potential to characterize microstructural features in human brain organoids modeling psychiatric disorders, neurodegenerative diseases, viral infections, and/or drug-induced neurotoxicity.
© 2024 The Authors.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

