Concizumab prophylaxis in persons with hemophilia A or B with inhibitors: patient-reported outcome results from the phase 3 explorer7 study
- PMID: 39099801
- PMCID: PMC11295565
- DOI: 10.1016/j.rpth.2024.102476
Concizumab prophylaxis in persons with hemophilia A or B with inhibitors: patient-reported outcome results from the phase 3 explorer7 study
Abstract
Background: Patient-reported outcomes (PROs) reflect patient perceptions of disease and treatment and are important for evaluating new therapies.
Objectives: Evaluate the effects of once-daily concizumab prophylaxis on health-related quality of life (HRQoL), treatment burden, and treatment preference in males aged ≥12 years with hemophilia A/B with inhibitors.
Methods: Patients enrolled in the multicenter, open-label explorer7 phase 3 study (ClinicalTrials.gov identifier: NCT04083781) were randomized to receive no prophylaxis (arm 1) or concizumab prophylaxis (arm 2) or were nonrandomly allocated to concizumab prophylaxis (arms 3 and 4). The study included questionnaires to assess patients' perception of HRQoL (Haemophilia Quality of Life Questionnaire for Adults), treatment burden (Hemophilia Treatment Experience Measure), and treatment preference (Haemophilia Patient Preference Questionnaire).
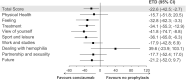
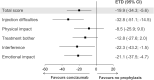
Results: The estimated treatment difference between patients receiving concizumab prophylaxis vs no prophylaxis at week 24 for Haemophilia Quality of Life Questionnaire for Adults "total score" was -22.6 points (95% CI, -42.5; -2.7), directionally favoring patients receiving concizumab prophylaxis. For Hemophilia Treatment Experience Measure "total score," the estimated treatment difference was -19.9 points (95% CI, -34.3, -5.6) in favor of concizumab vs no prophylaxis. The majority of patients receiving concizumab expressed a preference for concizumab over their previous treatment, the main reasons being "fewer bleeds," "require less time," and "less painful to inject." Across all PROs, there were less responses collected than anticipated, limiting interpretations.
Conclusion: PROs collected during the explorer7 study showed improvements in some domains of HRQoL, treatment burden, and patient treatment preference in persons with hemophilia A or B with inhibitors receiving concizumab prophylaxis compared with no prophylaxis.
Keywords: concizumab; health-related quality of life; hemophilia A; hemophilia B; patient preference; patient-reported outcomes.
© 2024 The Authors.
Figures


References
-
- Gringeri A., von Mackensen S. Quality of life in haemophilia. Haemophilia. 2008;14:19–25. - PubMed
-
- Srivastava A., Santagostino E., Dougall A., Kitchen S., Sutherland M., Pipe S.W., et al. WFH Guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26:1–158. - PubMed
-
- Ware J.E., Jr., Sherbourne C.D. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. - PubMed
-
- Wyrwich K.W., Krishnan S., Poon J.L., Auguste P., von Maltzahn R., Yu R., et al. Interpreting important health-related quality of life change using the Haem-A-QoL. Haemophilia. 2015;21:578–584. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

