Targeting Reprogrammed Cancer-Associated Fibroblasts with Engineered Mesenchymal Stem Cell Extracellular Vesicles for Pancreatic Cancer Treatment
- PMID: 39099892
- PMCID: PMC11293949
- DOI: 10.34133/bmr.0050
Targeting Reprogrammed Cancer-Associated Fibroblasts with Engineered Mesenchymal Stem Cell Extracellular Vesicles for Pancreatic Cancer Treatment
Abstract
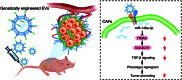
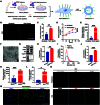
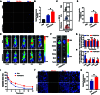
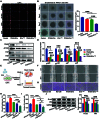
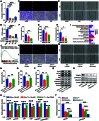
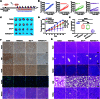
Background: As one of the most aggressive and lethal cancers, pancreatic cancer is highly associated with cancer-associated fibroblasts (CAFs) that influence the development and progression of cancer. Targeted reprogramming of CAFs may be a promising strategy for pancreatic cancer. This study aims to construct engineered extracellular vesicles (EVs) with surface modification of integrin α5 (ITGA5)-targeting peptide and high internal expression of miR-148a-3p by endogenous modification for targeted reprogramming of pancreatic CAFs. Methods: Bone marrow mesenchymal stem cells (BMSCs) and pancreatic CAFs were cocultured to examine the effect of BMSC-derived EVs on the expression levels of CAF markers. miR-148a-3p was identified as a functional molecule. The mechanism of miR-148a-3p was elucidated using the dual-luciferase reporter assay. BMSCs were infected with TERT-encoding and miR-148a-3p-encoding lentiviruses. Subsequently, BMSCs were modified with ITGA5-specific targeting peptide. The supernatant was ultracentrifuged to obtain the engineered EVs (ITGA5-EVs-148a), which were used to reprogram CAFs. Results: BMSCs modulated CAF marker expressions through EVs. miR-148a-3p was up-regulated in BMSCs. The expression of miR-148a-3p in pancreatic CAFs was down-regulated when compared with that in normal fibroblasts (NFs). Mechanistically, ITGA5-EVs-148a effectively suppressed the proliferation and migration of pancreatic CAFs by targeting ITGA5 through the TGF-β/SMAD pathway. ITGA5-EVs-148a was associated with enhanced cellular uptake and exhibited enhanced in vitro and in vivo targeting ability. Moreover, ITGA5-EVs-148a exerted strong reconfiguration effects in inactivating CAFs and reversing tumor-promoting effects in 3D heterospheroid and xenograft pancreatic cancer models. Conclusions: This targeted CAF reprogramming strategy with genetically engineered ITGA5-EVs-148a holds great promise as a precision therapeutics in clinical settings.
Copyright © 2024 Pengcheng Zhou et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H. The role of stroma in pancreatic cancer: Diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9(8):454–467. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

