How frequently are insects wounded in the wild? A case study using Drosophila melanogaster
- PMID: 39100166
- PMCID: PMC11296199
- DOI: 10.1098/rsos.240256
How frequently are insects wounded in the wild? A case study using Drosophila melanogaster
Abstract
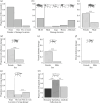
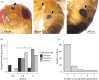
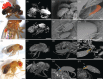
Wounding occurs across multicellular organisms. Wounds can affect host mobility and reproduction, with ecological consequences for competitive interactions and predator-prey dynamics. Wounds are also entry points for pathogens. An immune response is activated upon injury, resulting in the deposition of the brown-black pigment melanin in insects. Despite the abundance of immunity studies in the laboratory and the potential ecological and evolutionary implications of wounding, the prevalence of wounding in wild-collected insects is rarely systematically explored. We investigated the prevalence and potential causes of wounds in wild-collected Drosophilidae flies. We found that 31% of Drosophila melanogaster were wounded or damaged. The abdomen was the most frequently wounded body part, and females were more likely to have melanized patches on the ventral abdomen, compared with males. Encapsulated parasitoid egg frequency was approximately 10%, and just under 1% of Drosophilidae species had attached mites, which also caused wounds. Wounding is prevalent in D. melanogaster, likely exerting selection pressure on host immunity for two reasons: on a rapid and efficient wound repair and on responding efficiently to opportunistic infections. Wounding is thus expected to be an important driver of immune system evolution and to affect individual fitness and population dynamics.
Keywords: cuticle damage; cuticle injury; cuticle wound; melanin; mite; wild-collected organism.
© 2024 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures


Similar articles
-
An introduction to parasitic wasps of Drosophila and the antiparasite immune response.J Vis Exp. 2012 May 7;(63):e3347. doi: 10.3791/3347. J Vis Exp. 2012. PMID: 22588641 Free PMC article.
-
Endosymbiotic Male-Killing Spiroplasma Affects the Physiological and Behavioral Ecology of Macrocheles-Drosophila Interactions.Appl Environ Microbiol. 2022 Feb 8;88(3):e0197221. doi: 10.1128/AEM.01972-21. Epub 2021 Dec 8. Appl Environ Microbiol. 2022. PMID: 34878815 Free PMC article.
-
Recognition of nonself is necessary to activate Drosophila's immune response against an insect parasite.BMC Biol. 2024 Apr 22;22(1):89. doi: 10.1186/s12915-024-01886-1. BMC Biol. 2024. PMID: 38644510 Free PMC article.
-
Wounding in the plant tissue: the defense of a dangerous passage.Front Plant Sci. 2014 Sep 16;5:470. doi: 10.3389/fpls.2014.00470. eCollection 2014. Front Plant Sci. 2014. PMID: 25278948 Free PMC article. Review.
-
Evolution of Reproductive Behavior.Genetics. 2020 Jan;214(1):49-73. doi: 10.1534/genetics.119.302263. Genetics. 2020. PMID: 31907301 Free PMC article. Review.
Cited by
-
Juvenile responses to immune challenges are not carried through to subsequent life stages in an insect.Sci Rep. 2024 Sep 13;14(1):21456. doi: 10.1038/s41598-024-72546-1. Sci Rep. 2024. PMID: 39271717 Free PMC article.
-
Two matings lead to more copulatory wounding than a single mating in female Drosophila melanogaster.Proc Biol Sci. 2025 Jun;292(2049):20250523. doi: 10.1098/rspb.2025.0523. Epub 2025 Jun 18. Proc Biol Sci. 2025. PMID: 40527456 Free PMC article.
-
Insect immunity in the Anthropocene.Biol Rev Camb Philos Soc. 2025 Apr;100(2):698-723. doi: 10.1111/brv.13158. Epub 2024 Nov 5. Biol Rev Camb Philos Soc. 2025. PMID: 39500735 Free PMC article. Review.
References
-
- Fenner AL, Bull CM, Hutchinson MN. 2008. Injuries to lizards: conservation implications for the endangered pygmy bluetongue lizard (Tiliqua adelaidensis). Wildl. Res. 35 , 158. ( 10.1071/WR07103) - DOI
-
- Willis L, Threlkeld ST, Carpenter CC. 1982. Tail loss patterns in Thamnophis (Reptilia: Colubridae) and the probable fate of injured individuals. Copeia 1982 , 98. ( 10.2307/1444273) - DOI
-
- Plaistow SJ, Outreman Y, Moret Y, Rigaud T. 2003. Variation in the risk of being wounded: an overlooked factor in studies of invertebrate immune function? Ecol. Lett. 6 , 489–494. ( 10.1046/j.1461-0248.2003.00455.x) - DOI
Associated data
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

