Insights on Nefecon®, a Targeted-Release Formulation of Budesonide and Its Selective Immunomodulatory Effects in Patients with IgA Nephropathy
- PMID: 39100224
- PMCID: PMC11298173
- DOI: 10.2147/DDDT.S383138
Insights on Nefecon®, a Targeted-Release Formulation of Budesonide and Its Selective Immunomodulatory Effects in Patients with IgA Nephropathy
Abstract
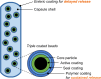
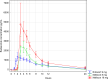
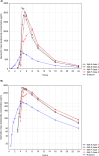
Immunoglobulin A nephropathy (IgAN) is a chronic, immune-mediated kidney disease characterized by the deposition of galactose-deficient immunoglobulin A1 (Gd-IgA1) in the kidneys. Excess Gd-IgA1 production in patients with IgAN is located within the mucosa-associated lymphoid tissue, particularly within the lamina propria in the distal ileum. Nefecon® is a targeted-release formulation of the corticosteroid budesonide, which became the first treatment approved by the US Food and Drug Administration (FDA; brand name, TARPEYO®) and European Medicines Agency (EMA; KINPEYGO®) for patients with primary IgAN at risk of rapid disease progression, after demonstrating clinically significant reduction of proteinuria in an interim analysis of the Phase III NefIgArd trial. After showing a significant reduction in estimated glomerular filtration rate decline in the full 2-year analysis of the trial, Nefecon was granted full approval by the FDA to reduce the loss of kidney function. Nefecon was specifically designed to deliver budesonide to the distal ileum, selectively targeting excess Gd-IgA1 production in the gut-associated lymphoid tissue. In this review, we describe the properties of Nefecon and the evidence to date that confirms its localized treatment effect. We also present unpublished evidence from Phase I trials investigating the pharmacokinetics and cortisol suppression effects of Nefecon in healthy participants. These studies demonstrated that Nefecon has a distinct pharmacokinetic profile from other budesonide products, allowing for targeted, localized action in the distal ileum. When considered alongside existing clinical trial data showing the effect of Nefecon on gut-associated biomarkers, available evidence indicates that Nefecon has a selective immunomodulatory mechanism of action and a direct disease-modifying effect in patients with IgAN, while having low systemic exposure and adverse effects.
Keywords: GALT; biomarkers; drug delivery; pharmacokinetics.
© 2024 Barratt et al.
Conflict of interest statement
JB is a consultant to Calliditas Therapeutics AB and reports grants and consultancy and personal fees from Calliditas Therapeutics, Everest Medicines, and STADA Arzneimittel AG. JK and MJ are consultants for Calliditas Therapeutics. CP is an employee of Calliditas Therapeutics. The authors report no other conflicts of interest in this work.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

