Dual-Target Additively Manufactured Electrochemical Sensor for the Multiplexed Detection of Protein A29 and DNA of Human Monkeypox Virus
- PMID: 39100359
- PMCID: PMC11292847
- DOI: 10.1021/acsomega.4c04460
Dual-Target Additively Manufactured Electrochemical Sensor for the Multiplexed Detection of Protein A29 and DNA of Human Monkeypox Virus
Abstract
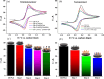
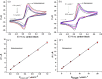
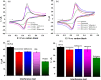
Herein, we present the first 3D-printed electrochemical portable biodevice for the detection of monkeypox virus (MKPV). The electrochemical device consists of two biosensors: an immunosensor and a genosensor specifically designed for the detection of the protein A29 and a target DNA of MKPV, respectively. The electrodes were manufactured using lab-made ultraflexible conductive filaments composed of carbon black, recycled PLA from coffee pods, and castor oil as a plasticizer. The sensors created through 3D printing technology exhibited good reproducibility and repeatability of analytical responses. Furthermore, both the immunosensor and genosensor demonstrated excellent MKPV detection capabilities, with a linear range from 0.01 to 1.0 μmol L-1 for the antigen and 0.1 to 20.0 μmol L-1 for the DNA target. The biosensors achieved limits of detection of 2.7 and 29 nmol L-1 for the immunosensor and genosensor, respectively. Interference tests conducted with the biosensors demonstrated their selectivity for MKPV. Moreover, analyses of fortified human serum samples showed recoveries close to 100%, confirming the absence of significant matrix effects for MKPV analysis. Therefore, the 3D-printed multiplex device represents a viable and highly promising alternative for on-site, portable, and rapid point-of-care MKPV monitoring.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Stefano J. S.; Silva L. R. G. e.; Kalinke C.; Oliveira P. R. de; Crapnell R. D.; Brazaca L. C.; Bonacin J. A.; Campuzano S.; Banks C. E.; Janegitz B. C. Human Monkeypox Virus: Detection Methods and Perspectives for Diagnostics. TrAC Trends Anal. Chem. 2023, 167, 117226 10.1016/j.trac.2023.117226. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
