Synthesis of N-Alkyl-3-[2-oxoquinolin-1(2 H)-yl]propanoic Acid Derivatives and Related Compounds: Cytotoxicity and EGFR Inhibition of Some Propanamide Derivatives
- PMID: 39100360
- PMCID: PMC11292662
- DOI: 10.1021/acsomega.4c03114
Synthesis of N-Alkyl-3-[2-oxoquinolin-1(2 H)-yl]propanoic Acid Derivatives and Related Compounds: Cytotoxicity and EGFR Inhibition of Some Propanamide Derivatives
Abstract
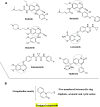
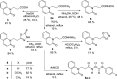
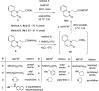
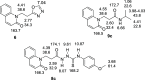
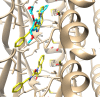
A series of 20 new structure-modified quinolin-2-one derivatives were prepared for biological evaluation. This was successfully achieved based on chemoselective reactions of heterocyclic amides with acrylic acid derivatives, which gave 3-[2-oxoquinolin-1-(2H)-yl] propanoic acid derivatives (N-substitution via a unique behavior). The ester was reacted with hydrazine to afford the corresponding hydrazide. Both the corresponding ester and hydrazide were used as building blocks to modify the quinolone structure and give N-hydroxyl propanamides, oxadiazoles, and thiosemicarbazides. The corresponding carboxylic acid and hydrazide were used to prepare several amides: N-alkyl-3-[2-oxoquinolin-1(2H)-yl]propanamides via azide and dicyclohexyl carbodiimide coupling methods. Among derivatives, compound 9e exhibited potent cytotoxicity against MCF-7 cells with an IC50 value of 1.32 μM compared to doxorubicin with an IC50 value of 1.21 μM. Additionally, it caused potent EGFR inhibition by 97% with an IC50 value of 16.89 nM compared to Erlotinib with an IC50 value of 29.8 nM. Finally, the binding mode of compound interactions toward EGFR was highlighted using a molecular docking study; compound 9e exhibited good binding affinity with a binding energy of -17.89 kcal/mol, and it formed H-bond interactions with Met 769 as the key amino acid of interaction. Accordingly, compound 9e may be developed as an EGFR-oriented chemotherapeutic antibreast cancer agent.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
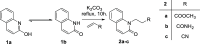

References
-
- Pavlović M. M.; Šeparović R.; Tečić V. A.; Vazdar L. Difference in Estimation of Side Effects of Chemotherapy between Physicians and Patients with Early-Stage Breast Cancer: The Use of Patient Reported Outcomes (PROs) in the Evaluation of Toxicity in Everyday Clinical Practice. Cancers 2021, 13, 5922.10.3390/cancers13235922. - DOI - PMC - PubMed
-
- Ilakiyalakshmi M.; Arumugam N. A. Review on recent development of quinoline for anticancer activities. Arab. J. Chem. 2022, 15, 104168.10.1016/j.arabjc.2022.104168. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
