iPSC-derived NK cells expressing high-affinity IgG Fc receptor fusion CD64/16A to mediate flexible, multi-tumor antigen targeting for lymphoma
- PMID: 39100677
- PMCID: PMC11294090
- DOI: 10.3389/fimmu.2024.1407567
iPSC-derived NK cells expressing high-affinity IgG Fc receptor fusion CD64/16A to mediate flexible, multi-tumor antigen targeting for lymphoma
Abstract
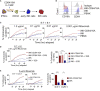
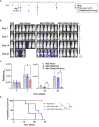
Introduction: NK cells can mediate tumor cell killing by natural cytotoxicity and by antibody-dependent cell-mediated cytotoxicity (ADCC), an anti-tumor mechanism mediated through the IgG Fc receptor CD16A (FcγRIIIA). CD16A polymorphisms conferring increased affinity for IgG positively correlate with clinical outcomes during monoclonal antibody therapy for lymphoma, linking increased binding affinity with increased therapeutic potential via ADCC. We have previously reported on the FcγR fusion CD64/16A consisting of the extracellular region of CD64 (FcγRI), a high-affinity Fc receptor normally expressed by myeloid cells, and the transmembrane/cytoplasmic regions of CD16A, to create a highly potent and novel activating fusion receptor. Here, we evaluate the therapeutic potential of engineered induced pluripotent stem cell (iPSC)-derived NK (iNK) cells expressing CD64/16A as an "off-the-shelf", antibody-armed cellular therapy product with multi-antigen targeting potential.
Methods: iNK cells were generated from iPSCs engineered to express CD64/16A and an interleukin (IL)-15/IL-15Rα fusion (IL-15RF) protein for cytokine independence. iNK cells and peripheral blood NK cells were expanded using irradiated K562-mbIL21-41BBL feeder cells to examine in in vitro and in vivo assays using the Raji lymphoma cell line. ADCC was evaluated in real-time by IncuCyte assays and using a xenograft mouse model with high circulating levels of human IgG.
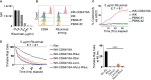
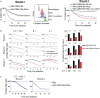
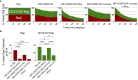
Results: Our data show that CD64/16A expressing iNK cells can mediate potent anti-tumor activity against human B cell lymphoma. In particular, (i) under suboptimal conditions, including low antibody concentrations and low effector-to-target ratios, iNK-CD64/16A cells mediate ADCC, (ii) iNK-CD64/16A cells can be pre-loaded with tumor-targeting antibodies (arming) to elicit ADCC, (iii) armed iNK-CD64/16A cells can be repurposed with additional antibodies to target new tumor antigens, and (iv) cryopreserved, armed iNK-CD64/16A are capable of sustained ADCC in a tumor xenograft model under saturating levels of human IgG.
Discussion: iNK-CD64/16A cells allow for a flexible use of antibodies (antibody arming and antibody targeting), and an "off-the-shelf" platform for multi-antigen recognition to overcome limitations of adoptive cell therapies expressing fixed antigen receptors leading to cancer relapse due to antigen escape variants.
Keywords: ADCC - antibody-dependent cellular cytotoxicity; antibody; cancer; immunotherapy; natural killer (NK) cell.
Copyright © 2024 Dixon, Snyder, Khaw, Hullsiek, Davis, Matson, Shirinbak, Hancock, Bjordahl, Hosking, Miller, Valamehr, Wu and Walcheck.
Conflict of interest statement
JW and BW are inventors on the patent application WO2019084388A1 Recombinant immune cells, methods of making, and methods of use. Human CD64/16A described in the patent application has been exclusively licensed to Fate Therapeutics. JSM is a paid consultant for Fate Therapeutics and JW, BW, and JSM receive research funds from Fate Therapeutics. Fate Therapeutics owns patent No. 10,626,372 Methods and compositions for inducing hematopoietic cell differentiation covering the iPSC derived NK cells. MH, RB, BH, SS, and BV are employees of Fate Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

