E-selectin in vascular pathophysiology
- PMID: 39100681
- PMCID: PMC11294169
- DOI: 10.3389/fimmu.2024.1401399
E-selectin in vascular pathophysiology
Abstract
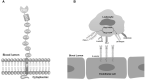
Selectins are a group of Ca2+-dependent, transmembrane type I glycoproteins which attract cell adhesion and migration. E-selectin is exclusively expressed in endothelial cells, and its expression is strongly enhanced upon activation by pro-inflammatory cytokines. The interaction of E-selectin with its ligands on circulating leukocytes captures and slows them down, further facilitating integrin activation, firm adhesion to endothelial cells and transmigration to tissues. Oxidative stress induces endothelial cell injury, leading to aberrant expression of E-selectin. In addition, the elevated level of E-selectin is positively related to high risk of inflammation. Dysregulation of E-selectin has been found in several pathological conditions including acute kidney injury (AKI), pulmonary diseases, hepatic pathology, Venous thromboembolism (VTE). Deletion of the E-selectin gene in mice somewhat ameliorates these complications. In this review, we describe the mechanisms regulating E-selectin expression, the interaction of E-selectin with its ligands, the E-selectin physiological and pathophysiological roles, and the therapeutical potential of targeting E-selectin.
Keywords: E-selectin; inflammation; oxidative stress; therapeutic interventions; vascular diseases.
Copyright © 2024 Zhang, Huang, Zhu, Gatt and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Boyle EM, Jr., Kovacich JC, Canty TG, Jr., Morgan EN, Chi E, Verrier ED, et al. . Inhibition of nuclear factor-kappa B nuclear localization reduces human E-selectin expression and the systemic inflammatory response. Circulation. (1998) 98:Ii282–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

