Effect of Peg-IFN on the viral kinetics of patients with HDV infection treated with bulevirtide
- PMID: 39100818
- PMCID: PMC11295569
- DOI: 10.1016/j.jhepr.2024.101070
Effect of Peg-IFN on the viral kinetics of patients with HDV infection treated with bulevirtide
Abstract
Background & aims: Bulevirtide is a first-in-class entry inhibitor antiviral treatment for chronic hepatitis D. The viral kinetics during bulevirtide therapy and the effect of combining bulevirtide with pegylated-interferon (Peg-IFN) are unknown.
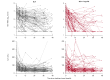
Methods: We used mathematical modelling to analyze the viral kinetics in two French observational cohorts of 183 patients receiving bulevirtide with or without Peg-IFN for 48 weeks.
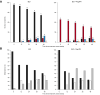
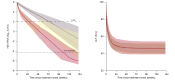
Results: The efficacy of bulevirtide in blocking cell infection was estimated to 90.3%, whereas Peg-IFN blocked viral production with an efficacy of 92.4%, albeit with large inter-individual variabilities. The addition of Peg-IFN to bulevirtide was associated with a more rapid virological decline, with a rate of virological response (>2 log of decline or undetectability) at week 48 of 86.9% (95% prediction interval [PI] = [79.7-95.0]), compared with 56.1% (95% PI = [46.4-66.7]) with bulevirtide only. The model was also used to predict the probability to achieve a cure of viral infection, with a rate of 8.8% (95% PI = [3.5-13.2]) with bulevirtide compared with 18.8% (95% PI = [11.6-29.0]) with bulevirtide + Peg-IFN. Mathematical modelling suggests that after 144 weeks of treatment, the rates of viral cure could be 42.1% (95% PI = [33.3-52.6]) with bulevirtide and 66.7% (95% PI = [56.5-76.8]) with bulevirtide + Peg-IFN.
Conclusions: In this analysis of real-world data, Peg-IFN strongly enhanced the kinetics of viral decline in patients treated with bulevirtide. Randomized clinical trials are warranted to assess the virological and clinical benefit of this combination, and to identify predictors of poor response to treatment.
Impact and implications: Bulevirtide has been approved for chronic HDV infection by regulatory agencies in Europe based on its good safety profile and rapid virological response after treatment initiation, but the optimal duration of treatment and the chance to achieve a sustained virological response remain unknown. The results presented in this study have a high impact for clinicians and investigators as they provide important knowledge on the long-term virological benefits of a combination of Peg-IFN and bulevirtide in patients with CHD. Clinical trials are now warranted to confirm those predictions.
Keywords: Antiviral treatment; Bulevirtide; Hepatitis delta virus; Peg-IFN; Viral kinetics.
© 2024 The Authors.
Conflict of interest statement
JG has received research funding and has consulted with Hoffman-LaRoche. SB, EG, and AG have received research funding from Eurobio Scientific. VdL received consulting fees from Gilead, AbbVie, BMS, GSK, Escopics, Alfasigma, Janssen, Orphalan, NovoNordisk, AstraZeneca. JMP has served as an advisor or speaker for Abbott, Abbvie, Gilead, and GSK. The other authors have no conflicts of interest to declare. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- Global progress report on HIV viral Hepat Sex Transm infections. 2021 https://www.who.int/publications-detail-redirect/9789240027077
-
- Farci P. Delta hepatitis: an update. J Hepatol. 2003;39:212–219. - PubMed
-
- Niro G.A., Gioffreda D., Fontana R. Hepatitis delta virus infection: open issues. Dig Liver Dis. 2011;43:S19–S24. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

