Luteolin Alleviates Diabetic Nephropathy Fibrosis Involving AMPK/NLRP3/TGF-β Pathway
- PMID: 39100967
- PMCID: PMC11297584
- DOI: 10.2147/DMSO.S450094
Luteolin Alleviates Diabetic Nephropathy Fibrosis Involving AMPK/NLRP3/TGF-β Pathway
Abstract
Purpose: Luteolin is a promising candidate for diabetic nephropathy due to its potential anti-inflammatory and anti-fibrotic properties. This study explored the molecular mechanisms through which luteolin combats fibrosis in DN.
Methods: Potential targets affected by luteolin and genes associated with DN were collected from databases. Overlapping targets between luteolin and diabetic nephropathy were identified through Venn analysis. A protein-protein interaction network was constructed using these common targets, and critical pathways and targets were elucidated through GO and KEGG analysis. These pathways and targets were confirmed using a streptozotocin-induced mouse model. Luteolin was administered at 45 mg/kg and 90 mg/kg. Various parameters were evaluated, including body weight, blood glucose levels, and histopathological examinations. Protein levels related to energy metabolism, inflammation, and fibrosis were quantified.
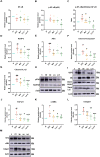
Results: Fifty-three targets associated with luteolin and 36 genes related to diabetic nephropathy were extracted. The AGE-RAGE signaling pathway was the key pathway impacted by luteolin in diabetic nephropathy. Key molecular targets include TGF-β, IL-1β, and PPARG. Luteolin reduced body weight and blood glucose levels, lowered the left kidney index, and improved insulin and glucose tolerance. Furthermore, luteolin mitigated inflammatory cell infiltration, basement membrane thickening, and collagen deposition in the kidney. Luteolin up-regulated the protein expression of p-AMPKα (Th172) while simultaneously down-regulated the protein expression of p-NF-ĸB (p65), NLRP3, TGF-β1, α-SMA, and Collagen I.
Conclusion: Luteolin mitigated renal fibrosis by alleviating energy metabolism disruptions and inflammation by modulating the AMPK/NLRP3/TGF-β signaling pathway.
Keywords: TGF-β; diabetic nephropathy; fibrosis; inflammation; network pharmacology.
© 2024 Huang et al.
Conflict of interest statement
The authors report no potential conflicts of interest in this work.
Figures






References
-
- Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in u.s. Adult patients with type 1 diabetes: a real-world study. Diabetes Care. 2019;42(12):2220–2227. doi: 10.2337/dc19-0830 - DOI - PubMed
-
- Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (sglt-2) inhibitors and glucagon-like peptide-1 (glp-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

