pH and thiosulfate dependent microbial sulfur oxidation strategies across diverse environments
- PMID: 39101034
- PMCID: PMC11294248
- DOI: 10.3389/fmicb.2024.1426584
pH and thiosulfate dependent microbial sulfur oxidation strategies across diverse environments
Abstract
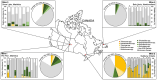
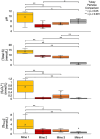
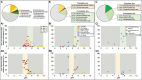
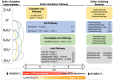
Sulfur oxidizing bacteria (SOB) play a key role in sulfur cycling in mine tailings impoundment (TI) waters, where sulfur concentrations are typically high. However, our understanding of SOB sulfur cycling via potential S oxidation pathways (sox, rdsr, and S4I) in these globally ubiquitous contexts, remains limited. Here, we identified TI water column SOB community composition, metagenomics derived metabolic repertoires, physicochemistry, and aqueous sulfur concentration and speciation in four Canadian base metal mine, circumneutral-alkaline TIs over four years (2016 - 2019). Identification and examination of genomes from nine SOB genera occurring in these TI waters revealed two pH partitioned, metabolically distinct groups, which differentially influenced acid generation and sulfur speciation. Complete sox (csox) dominant SOB (e.g., Halothiobacillus spp., Thiomonas spp.) drove acidity generation and S2O3 2- consumption via the csox pathway at lower pH (pH ~5 to ~6.5). At circumneutral pH conditions (pH ~6.5 to ~8.5), the presence of non-csox dominant SOB (hosting the incomplete sox, rdsr, and/or other S oxidation reactions; e.g. Thiobacillus spp., Sulfuriferula spp.) were associated with higher [S2O3 2-] and limited acidity generation. The S4I pathway part 1 (tsdA; S2O3 2- to S4O6 2-), was not constrained by pH, while S4I pathway part 2 (S4O6 2- disproportionation via tetH) was limited to Thiobacillus spp. and thus circumneutral pH values. Comparative analysis of low, natural (e.g., hydrothermal vents and sulfur hot springs) and high (e.g., Zn, Cu, Pb/Zn, and Ni tailings) sulfur systems literature data with these TI results, reveals a distinct TI SOB mining microbiome, characterized by elevated abundances of csox dominant SOB, likely sustained by continuous replenishment of sulfur species through tailings or mining impacted water additions. Our results indicate that under the primarily oxic conditions in these systems, S2O3 2- availability plays a key role in determining the dominant sulfur oxidation pathways and associated geochemical and physicochemical outcomes, highlighting the potential for biological management of mining impacted waters via pH and [S2O3 2-] manipulation.
Keywords: pH; sox genes; sulfur oxidizing bacteria (SOB); tailings impoundments; thiosulfate.
Copyright © 2024 Twible, Whaley-Martin, Chen, Colenbrander Nelson, Arrey, Jarolimek, King, Ramilo, Sonnenberg, Banfield, Apte and Warren.
Conflict of interest statement
LR and HS were employed by EcoReg Solutions. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Akcil A., Koldas S. (2006). Acid mine drainage (AMD): causes, treatment and case studies. J. Clean. Prod. 14, 1139–1145. doi: 10.1016/J.JCLEPRO.2004.09.006 - DOI
-
- Arce-Rodríguez A., Puente-Sánchez F., Avendaño R., Martínez-Cruz M., de Moor J. M., Pieper D. H., et al. . (2019). Thermoplasmatales and sulfur-oxidizing bacteria dominate the microbial community at the surface water of a CO 2-rich hydrothermal spring located in Tenorio volcano national park. Costa Rica. Extremophiles 23, 177–187. doi: 10.1007/s00792-018-01072-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

