pH-induced morphological transition of aggregates formed by miktoarm star polymers in dilute solution: a mesoscopic simulation study
- PMID: 39101066
- PMCID: PMC11295911
- DOI: 10.1039/d4ra04511d
pH-induced morphological transition of aggregates formed by miktoarm star polymers in dilute solution: a mesoscopic simulation study
Abstract
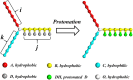
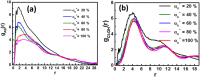
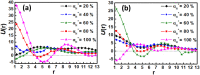
The self-assembly of miktoarm star polymers μ-A i (B(D)) j C k in a neutral solution and the pH-responsive behaviors of vesicles and spherical micelles in an acidic solution have been investigated by DPD simulation. The results show that the self-assembled morphologies can be regulated by the lengths of pH-responsive arm B and hydrophilic arm C, leading to the formation of vesicles, discoidal micelles, and spherical micelles in a neutral solution. The dynamic evolution pathways of vesicles and spherical micelles are categorized into three stages: nucleation, coalescence, and growth. Subsequently, the pH-responsive behaviors of vesicles and spherical micelles have been explored by tuning the protonation degree of pH-responsive arm B. The vesicles evolves from nanodisks to nanosheets, then to nanoribbons, as the protonation degree increases, corresponding to a decrease in pH value, while the spherical micelles undergoes a transition into worm-like micelles, nanosheets, and nanoribbons. Notably, the electrostatic interaction leads the counterions to form a regular hexagonal pattern in nanosheets, while an alternative distribution of charged beads has been observed in nanoribbons. Furthermore, the role of the electrostatic interaction in the morphological transition has been elucidated through the analysis of the distribution of positive and negative charges, as well as the electrostatic potential for associates.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Khanna K. Varshney S. Kakkar A. Polym. Chem. 2010;1:1171–1185.
-
- Yang L. Hu X. Wang W. Liu S. Sun T. Huang Y. Jing X. Xie Z. RSC Adv. 2014;4:41588–41596.
-
- Ren J. M. McKenzie T. G. Fu Q. Wong E. H. H. Xu J. An Z. Shanmugam S. Davis T. P. Boyer C. Qiao G. G. Chem. Rev. 2016;116:6743–6836. - PubMed
-
- Aghajanzadeh M. Zamani M. Rostamizadeh K. Sharafi A. Danafar H. J. Macromol. Sci., Part A: Pure Appl. Chem. 2018;55:559–571.
-
- Ding H. Tan P. Fu S. Tian X. Zhang H. Ma X. Gu Z. Luo K. J. Controlled Release. 2022;348:206–238. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

