Dapagliflozin Enhances Arterial and Venous Compliance During Exercise in Heart Failure With Preserved Ejection Fraction: Insights From the CAMEO-DAPA Trial
- PMID: 39101201
- PMCID: PMC11433515
- DOI: 10.1161/CIRCULATIONAHA.124.068788
Dapagliflozin Enhances Arterial and Venous Compliance During Exercise in Heart Failure With Preserved Ejection Fraction: Insights From the CAMEO-DAPA Trial
Abstract
Background: Systemic arterial compliance and venous capacitance are typically impaired in patients with heart failure with preserved ejection fraction (HFpEF), contributing to hemodynamic congestion with stress. Sodium-glucose cotransporter-2 inhibitors reduce hemodynamic congestion and improve clinical outcomes in patients with HFpEF, but the mechanisms remain unclear. This study tested the hypothesis that Dapagliflozin would improve systemic arterial compliance and venous capacitance during exercise in patients with HFpEF.
Methods: In this secondary analysis from the CAMEO-DAPA trial (Cardiac and Metabolic Effects of Dapagliflozin in Heart Failure With Preserved Ejection Fraction Trial), 37 patients with HFpEF (mean age 68 ± 9 years, women 65%) underwent invasive hemodynamic exercise testing with simultaneous echocardiography at baseline and following treatment for 24 weeks with Dapagliflozin or placebo. Radial artery pressure (BP) was measured continuously using a fluid-filled catheter with transformation to aortic pressure, central hemodynamics were measured using high-fidelity micromanometers, and stressed blood volume was estimated from hemodynamic indices fit to a comprehensive cardiovascular model.
Results: There was no statistically significant effect of Dapagliflozin on resting BP, but Dapagliflozin reduced systolic BP during peak exercise (estimated treatment difference [ETD], -18.8 mm Hg [95% CI, -33.9 to -3.7] P=0.016). Reduction in BP was related to improved exertional total arterial compliance (ETD, 0.06 mL/mm Hg/m2 [95% CI, 0.003-0.11] P=0.039) and aortic root characteristic impedance (ETD, -2.6 mm Hg/mL*sec [95% CI: -5.1 to -0.03] P=0.048), with no significant effect on systemic vascular resistance. Dapagliflozin reduced estimated stressed blood volume at rest and during peak exercise (ETD, -292 mm Hg [95% CI, -530 to -53] P=0.018), and improved venous capacitance evidenced by a decline in ratio of estimated stressed blood volume to total blood volume (ETD, -7.3% [95% CI, -13.3 to -1.3] P=0.020). Each of these effects of Dapagliflozin at peak exercise were also observed during matched 20W exercise intensity. Improvements in total arterial compliance and estimated stressed blood volume were correlated with decreases in body weight, and reduction in systolic BP with treatment was correlated with the change in estimated stressed blood volume during exercise (r=0.40, P=0.019). Decreases in BP were correlated with reduction in pulmonary capillary wedge pressure during exercise (r=0.56, P<0.001).
Conclusions: In patients with HFpEF, treatment with Dapagliflozin improved systemic arterial compliance and venous capacitance during exercise, while reducing aortic characteristic impedance, suggesting a reduction in arterial wall stiffness. These vascular effects may partially explain the clinical benefits with sodium-glucose cotransporter-2 inhibitors in HFpEF.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04730947.
Keywords: Dapagliflozin; arterial compliance; exercise; heart failure, diastolic; hemodynamics; vascular stiffness; venous function.
Conflict of interest statement
Dr Borlaug receives research support from the National Institutes of Health (NIH) and the United States Department of Defense, as well as research grant funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, and Tenax Therapeutics. Dr Borlaug has served as a consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, NGM, NXT, and VADovations, and is named inventor (US Patent no. 10,307,179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure, Dr Chirinos has recently consulted for Bayer, Fukuda-Denshi, Bristol-Myers Squibb, JNJ, Edwards Life Sciences, Merck, and NGM Biopharmaceuticals. He received University of Pennsylvania research grants from National Institutes of Health, Fukuda-Denshi, Bristol-Myers Squibb, Microsoft and Abbott. He is named as inventor in a University of Pennsylvania patent for the use of inorganic nitrates/nitrites for the treatment of HFpEF and for the use of biomarkers in heart failure. He has received research device loans from Atcor Medical, Fukuda-Denshi, Unex, Uscom, NDD Medical Technologies, Microsoft and MicroVision Medical. B. Pourmussa has no relevant disclosures.
Figures
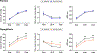
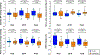
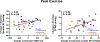
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- K23 HL164901/HL/NHLBI NIH HHS/United States
- R01 HL162828/HL/NHLBI NIH HHS/United States
- R01 HL155599/HL/NHLBI NIH HHS/United States
- R01 HL157108/HL/NHLBI NIH HHS/United States
- K24 AG070459/AG/NIA NIH HHS/United States
- R01 HL104106/HL/NHLBI NIH HHS/United States
- R01 AG058969/AG/NIA NIH HHS/United States
- R01 HL153646/HL/NHLBI NIH HHS/United States
- U01 HL160277/HL/NHLBI NIH HHS/United States
- R33 HL146390/HL/NHLBI NIH HHS/United States
- U01 HL160226/HL/NHLBI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 HL155764/HL/NHLBI NIH HHS/United States
- R01 HL128526/HL/NHLBI NIH HHS/United States
- U01 TR003734/TR/NCATS NIH HHS/United States

