A novel liposomal formulation for ocular delivery of caspofungin: an experimental study by quality by design-based approach
- PMID: 39101438
- PMCID: PMC11415022
- DOI: 10.1080/20415990.2024.2379756
A novel liposomal formulation for ocular delivery of caspofungin: an experimental study by quality by design-based approach
Abstract
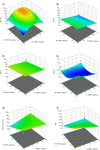
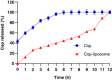
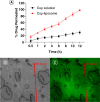
Aim: This study focuses on the development of a Caspofungin liposome for efficient ocular delivery by enhancing corneal penetration.Method: Quality by design (QbD) approach was adopted to identify critical factors that influence final liposomal formulation. The liposome developed using thin film hydration after optimization was subjected to characterization for physicochemical properties, irritation potential and corneal uptake.Results: The numerical optimization suggests an optimal formulation with a desirability value of 0.706, using CQAs as optimization goals with 95% prediction intervals. The optimized formulation showed no signs of irritation potential along with observation of significant corneal permeation.Conclusion: The liposomal formulation increased the permeability of Caspofungin, which could enhance the efficacy for the treatment of conditions, like fungal keratitis.
Keywords: Caspofungin; echinocandins; fungal keratitis; liposome; ocular delivery; quality by design.
Plain language summary
[Box: see text].
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
