Gemogenovatucel-T (Vigil): bi-shRNA plasmid-based targeted immunotherapy
- PMID: 39101448
- PMCID: PMC11509044
- DOI: 10.1080/14796694.2024.2376518
Gemogenovatucel-T (Vigil): bi-shRNA plasmid-based targeted immunotherapy
Abstract
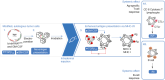
We describe in this review the historical evidence leading up to the concept and design of Vigil and subsequent clinical applications including safety and efficacy in a randomized, controlled Phase IIB trial. Vigil (gemogenovatucel-T) is a unique triple function targeted immunotherapy that demonstrates preclinical and clinical systemic anticancer activity. Construction of Vigil involves harvest of autologous malignant tissue for neoantigen targeting (ideally containing clonal neoantigens) followed by a two-day process involving transfection with a plasmid to provide a permissive 'training environment' for the patient's immune system. Transfected plasmid components contain an expressive human GMCSF DNA segment to enhance anticancer immune functional response and a second component expressing bi-shRNAfurin which reduces TGFβ isomers (TGFβ1 and TGFβ2) thereby reducing cancer inhibition of the targeted immune response. Results generated to date justify advancement to confirmatory clinical trials supporting product regulatory approval.
Keywords: anticancer; bi-shRNA; cancer; clonal neoantigen; immunotherapy; ovarian; plasmid; vigil.
Plain language summary
Vigil is an anticancer treatment that employs three methods of enhancing the body's immune system to identify and kill cancer cells. The construction of Vigil involves cancer cells from the same person being treated (personalized therapy) in combination with added anticancer genetic signals to enhance the number and function anti-anticancer immune cells and to guide the immune cells to the cancer and not to normal organs of the body. In this manner, an army of immune cells are created that can move to attacking the cancer using blood vessels to get to the cancer anywhere it tries to grow in the body. One study (Phase I) performed with this product to determine safety and dose range demonstrated an optimal dose and schedule. Another study (Phase IIA) showed initial clinical benefit. A third more complex study (Phase IIB) in patients treated with Vigil compared with standard of care without Vigil demonstrated the ability to prolong the patients life and time without their cancer getting worse without any significant side effects associated with the treatment in a unique subset of ovarian cancer patients, those with the ability to repair their DNA. Based on the composite of these results, Vigil is an attractive targeted immunotherapy justified for late-stage clinical testing.
Conflict of interest statement
J Nemunaitis and S Engle report employment, stockholder and board membership of Gradalis, Inc. L Stanbery and A Walter are employees of Gradalis, Inc. G Wallraven, S Horvath, E Bognar and D Rao report stockholder and employee of Gradalis, Inc. S Brun reports being a paid consultant for Gradalis, Inc. RP Rocconi reports being paid consultant for Gradalis, Inc, ImmunoGen, Easai and GSK. KC has no conflict of interest to declare. BJ Monk reports being a paid consultant for Acrivon, Adaptimmune, Agenus, Akeso Bio, Amgen, Aravive, AstraZeneca, Bayer, Clovis, Easai, Elevar, EMD Merck, Genmab/Seagen, GOG Foundation, Gradalis, Inc, Heng Rui, ImmunoGen, Karyopharm, Iovance, Laekna Health Care, Merck, Mersana, Myriad, Novartis, Novocure, OncoC4, Panavance, Pieris, Pfizer, Puma, Regeneron, Roche/Genentech, Sorrento, TESARO/GSK, US Oncology Research, VBL, Verastem, Zentalis. RL Coleman reports grants and contracts from AstraZeneca, Clovis, Genelux, Genmab, Merck, Immunogen, Roche/Genentech; paid consultant for Agenus, Alkermes, AstraZeneca, Clovis, Deciphera, Genelux, Genmab, GSK, Immunogen, OncoQuest, Onxerna, Regeneron, Roche/Genentech, Novocure, Merck, Abbvie; participation on a data safety monitoring board or advisory board for NRG Oncology and Eisai/BMS. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures



References
-
- Rocconi RP, Grosen EA, Ghamande SA, et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): a randomised, double-blind, placebo-controlled, Phase IIB trial. Lancet Oncol. 2020;21(12):1661–1672. doi: 10.1016/S1470-2045(20)30533-7 - DOI - PubMed
-
•• Reports VITAL study results.
-
- Walter A, Rocconi RP, Monk BJ, et al. Gemogenovatucel-T (Vigil) maintenance immunotherapy: 3-year survival benefit in homologous recombination proficient (HRP) ovarian cancer. Gynecol Oncol. 2021;163(3):459–464. doi: 10.1016/j.ygyno.2021.10.004 - DOI - PubMed
-
•• Follow up of survival benefit in patients receiving Vigil who are HRP molecular profile.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
