New insights on hidradenitis suppurativa phenotypes and treatment response: An exploratory automated analysis of the SUNSHINE and SUNRISE trials
- PMID: 39101698
- PMCID: PMC12290999
- DOI: 10.1111/jdv.20234
New insights on hidradenitis suppurativa phenotypes and treatment response: An exploratory automated analysis of the SUNSHINE and SUNRISE trials
Abstract
Background: Defining hidradenitis suppurativa (HS) subtypes was previously limited by small sample sizes and poor interrater reliability; no study has investigated subtype treatment responses. The objective of this analysis was to characterize HS clusters in adult patients with moderate to severe HS and evaluate secukinumab treatment responses between clusters.
Methods: Clusters were identified via an unsupervised machine learning clustering analysis using baseline data from the randomized, placebo-controlled SUNSHINE (NCT03713619) and SUNRISE (NCT03713632) phase 3 trials. To assess treatment responses, patients received secukinumab every 2 (SECQ2W) or 4 weeks (SECQ4W) or placebo, for 16 weeks, after which, placebo patients randomly switched to SECQ2W/SECQ4W, and SECQ2W/SECQ4W patients maintained their original treatment, until week 52. Baseline outcomes included patient characteristics, disease characteristics and severity, HS-associated comorbidities and previous treatment exposures. Treatment response was assessed via the HS clinical response (HiSCR), abscess and inflammatory nodule (AN) count, flares and NRS30 (skin pain).
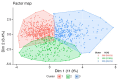
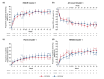
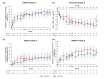
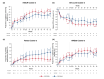
Results: Based on baseline data, three clusters were identified from 1084 patients (Cluster 1: 54.1%, Cluster 2: 17.8%, Cluster 3: 28.1%). Cluster 1 was predominantly female (65.4%) and was characterized by milder HS. Cluster 2 had more patients from the Asia Pacific, Middle East and Africa region (58.5%) and was characterized by moderate HS. Cluster 3 had the highest rates of previous exposure to biologics (45.9%) and prior HS-related surgeries (47.5%) and was characterized by severe HS. SECQ2W and SECQ4W demonstrated efficacy versus placebo in all clusters at week 16; SECQ2W and SECQ4W efficacy was maintained to week 52. SECQ2W treatment showed a trend for greater efficacy versus SECQ4W in Cluster 3 through week 52.
Conclusions: Three HS clusters were identified. Secukinumab demonstrated benefit over placebo in all clusters. However, patients with more severe disease may take longer to respond and more frequent secukinumab dosing may be required for these patients.
Trial registration: SUNSHINE (NCT03713619) and SUNRISE (NCT03713632).
© 2024 The Author(s). Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Conflict of interest statement
Anna Passera and David Demanse are employees and stockholders at Novartis Pharma AG, Basel, Switzerland. Lorenz Uhlmann and Marc Vandemeulebroecke were full‐time employees at the time the research was conducted and are current stockholders of Novartis Pharma AG, Basel, Switzerland. Ginette A. Okoye serves on the Board of Directors for the Hidradenitis Suppurativa Foundation; served as a consultant for Unilever; has served on advisory boards for AbbVie, Janssen, Lilly, Novartis, Pfizer, and UCB and has received fellowship grants from Janssen and Pfizer. Gregor B. E. Jemec has served as a consultant for AbbVie, Coloplast, Leo Pharma, Novartis, UCB and InflaRX; as an investigator for AbbVie, Leo Pharma, Novartis, Regeneron, UCB and InflaRX; received unrestricted grants from AbbVie, Leo Pharma and Novartis; served on Adboards for AbbVie, Janssen‐Pharma, MSD and Novartis and as a speaker for AbbVie, Coloplast, Leo Pharma and Galderma. Tiffany Mayo has served as an investigator or consultant for Arcutis, Acelyrin, BMS, ChemoCentryx, Eli Lilly, Galderma, Janssen, Leo Pharma, Novartis, Pfizer and Procter and Gamble. Jennifer Hsiao serves on the Board of Directors for the Hidradenitis Suppurativa Foundation, has served as an investigator or consultant for Aclaris Therapeutics, Amgen, Boehringer Ingelheim, Incyte, Novartis and UCB and has served as a speaker for AbbVie. Vivian Shi serves on the board of directors for the Hidradenitis Suppurativa Foundation, is an advisor for the National Eczema Association, is a stock shareholder of Learn Health and has served as an advisory board member, investigator, speaker and/or received research funding from Sanofi Genzyme, Regeneron, AbbVie, Eli Lilly, Novartis, SUN Pharma, LEO Pharma, Pfizer, Incyte, Boehringer Ingelheim, Genentech, Alumis Aristea Therapeutics, Menlo Therapeutics, Dermira, Burt's Bees, Galderma, Kiniksa, UCB, Target‐PharmaSolutions, Altus Lab/cQuell, MYOR, Polyfins Technology, GpSkin and Skin Actives Scientific. Angel S. Byrd is a consultant for Senté and Sonoma Biotherapeutics and participated in the Novartis HS advisory board (Miami, FL, USA). She is the inaugural recipient of the Skin of Colour Society Career Development Award as well as the Society for Investigative Dermatology Freinkel Diversity Fellowship Award and a recipient of the Robert A. Winn Diversity in Clinical Trials Career Development Award (Winn CDA) funded by Bristol Myers Squibb Foundation (BMSF). Xiaoling Wei is an employee at Novartis Pharma Shanghai, China. Shoba Ravichandran and Elisa Muscianisi are employees and stockholders at Novartis Pharmaceuticals Corporation, East Hanover, USA and Novartis Gene Therapies, Bannockburn, Illinois, USA, respectively. Martina J. Porter is a consultant and/or investigator for AbbVie, Janssen, Novartis, Sonoma Biotherapeutics, Regeneron, Aristea Therapeutics, Moonlake, Acelyrin, Anaptys bio, Pfizer, UCB, Eli Lilly, Alumis and Incyte.
Figures
References
-
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 Suppl 1):S4–S7. - PubMed
-
- Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis suppurativa/acne Inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231(2):184–190. - PubMed
-
- Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318(20):2019–2032. - PubMed
-
- Prens E, Deckers I. Pathophysiology of hidradenitis suppurativa: an update. J Am Acad Dermatol. 2015;73(5 Suppl 1):S8–S11. - PubMed
-
- Zouboulis CC, Bechara FG, Dickinson‐Blok JL, Gulliver W, Horváth B, Hughes R, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization – systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. 2019;33(1):19–31. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

