Redirecting B7-H3.CAR T Cells to Chemokines Expressed in Osteosarcoma Enhances Homing and Antitumor Activity in Preclinical Models
- PMID: 39101835
- PMCID: PMC11443211
- DOI: 10.1158/1078-0432.CCR-23-3298
Redirecting B7-H3.CAR T Cells to Chemokines Expressed in Osteosarcoma Enhances Homing and Antitumor Activity in Preclinical Models
Abstract
Purpose: Clinical efficacy of chimeric antigen receptor (CAR) T cells against pediatric osteosarcoma (OS) has been limited. One strategy to improve efficacy may be to drive chemokine-mediated homing of CAR T cells to tumors. We sought to determine the primary chemokines secreted by OS and evaluate the efficacy of B7-H3.CAR T cells expressing the cognate receptors.
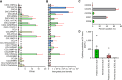
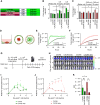
Experimental design: We developed a pipeline to identify chemokines secreted by OS by correlating RNA-seq data with chemokine protein detected in media from fresh surgical specimens. We identified CXCR2 and CXCR6 as promising receptors for enhancing CAR T-cell homing against OS. We evaluated the homing kinetics and efficiency of CXCR2- and CXCR6.T cells and homing, cytokine production, and antitumor activity of CXCR2- and CXCR6.B7-H3.CAR T cells in vitro and in vivo.
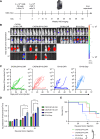
Results: T cells transgenically expressing CXCR2 or CXCR6 exhibited ligand-specific enhanced migration over T cells modified with nonfunctional control receptors. Differential homing kinetics were observed, with CXCR2.T-cell homing quickly and plateauing early, whereas CXCR6.T cells took longer to home but achieved a similar plateau. When expressed in B7-H3.CAR T cells, CXCR2- and CXCR6 modification conferred enhanced homing toward OS in vitro and in vivo. CXCR2- and CXCR6-B7-H3.CAR-treated mice experienced prolonged survival in a metastatic model compared with B7-H3.CAR T-cell-treated mice.
Conclusions: Our patient-based pipeline identified targets for chemokine receptor modification of CAR T cells targeting OS. CXCR2 and CXCR6 expression enhanced the homing and anti-OS activity of B7-H3.CAR T cells. These findings support clinical evaluation of CXCR-modified CAR T cells to improve adoptive cell therapy for patients with OS.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
L.J. Talbot reports grants from National Cancer Institute, Assisi Foundation of Memphis, Rally Foundation, and Infinite Love Foundation during the conduct of the study. P. Nguyen reports a patent for B7-H3 Chimeric Antigen Receptors, US Application 17/917,198 pending. P.J. Chockley reports a patent for CAR designs for immunotherapy pending. S. Gottschalk reports grants and other support from American Lebanese Syrian Associated Charities during the conduct of the study and personal fees from Tessa Therapeutics, Immatics, Tidal, Cargo, and Be Biopharma outside the submitted work, as well as multiple pending patents in the fields of T-cell, NK-cell, or gene therapy. C. DeRenzo reports grants from the Assisi Foundation of Memphis during the conduct of the study, as well as a patent for B7-H3 Chimeric Antigen Receptors, US application 17/917,198 pending. No disclosures were reported by the other authors.
Figures



References
-
- Lagmay JP, Krailo MD, Dang H, Kim A, Hawkins DS, Beaty O III, et al. Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through children's cancer group, pediatric oncology group, and children's oncology group: learning from the past to move forward. J Clin Oncol 2016;34:3031–8. - PMC - PubMed
-
- Kleinerman E. Maximum benefit of chemotherapy for osteosarcoma achieved-what are the next steps? Lancet Oncol 2016;17:1340–2. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

