Unveiling hierarchy and spatial distribution of O6-methylguanine-DNA methyltransferase promoter methylation in World Health Organization grade 2-3 gliomas
- PMID: 39101880
- PMCID: PMC11447971
- DOI: 10.1111/cas.16268
Unveiling hierarchy and spatial distribution of O6-methylguanine-DNA methyltransferase promoter methylation in World Health Organization grade 2-3 gliomas
Abstract
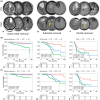
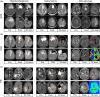
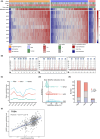
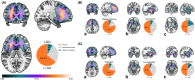
This study investigated the role of O6-methylguanine-DNA methyltransferase promoter (MGMTp) methylation hierarchy and heterogeneity in grade 2-3 gliomas, focusing on variations in chemotherapy benefits and resection dependency. A cohort of 668 newly diagnosed grade 2-3 gliomas, with comprehensive clinical, radiological, and molecular data, formed the basis of this analysis. The extent of resection was categorized into gross total resection (GTR ≥100%), subtotal resection (STR >90%), and partial resection (PR ≤90%). MGMTp methylation levels were examined using quantitative pyrosequencing. Our findings highlighted the critical role of GTR in improving the prognosis for astrocytomas (IDH1/2-mutant and 1p/19q non-codeleted), contrasting with its lesser significance for oligodendrogliomas (IDH1/2 mutation and 1p/19q codeletion). Oligodendrogliomas demonstrated the highest average MGMTp methylation levels (median: 28%), with a predominant percentage of methylated cases (average methylation levels >20%). Astrocytomas were more common in the low-methylated group (10%-20%), while IDH wild-type gliomas were mostly unmethylated (<10%). Spatial distribution analysis revealed a decrement in frontal lobe involvement from methylated, low-methylated to unmethylated cases (72.8%, 59.3%, and 47.8%, respectively). In contrast, low-methylated and unmethylated cases were more likely to invade the temporal-insular region (19.7%, 34.3%, and 40.4%, respectively). Astrocytomas with intermediate MGMTp methylation were notably associated with temporal-insular involvement, potentially indicating a moderate response to temozolomide and underscoring the importance of aggressive resection strategies. In conclusion, our study elucidates the complex interplay of MGMTp methylation hierarchy and heterogeneity among grade 2-3 gliomas, providing insights into why astrocytomas and IDH wild-type lower-grade glioma might derive less benefit from chemotherapy.
Keywords: MGMT promoter; astrocytoma; grade 2–3 gliomas; hierarchy; oligodendroglioma.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures
Similar articles
-
The interplay mechanism between IDH mutation, MGMT-promoter methylation, and PRMT5 activity in the progression of grade 4 astrocytoma: unraveling the complex triad theory.Oncol Res. 2024 May 23;32(6):1037-1045. doi: 10.32604/or.2024.051112. eCollection 2024. Oncol Res. 2024. PMID: 38827324 Free PMC article.
-
TERT promoter mutation confers favorable prognosis regardless of 1p/19q status in adult diffuse gliomas with IDH1/2 mutations.Acta Neuropathol Commun. 2020 Nov 23;8(1):201. doi: 10.1186/s40478-020-01078-2. Acta Neuropathol Commun. 2020. PMID: 33228806 Free PMC article.
-
Molecular prognostic factors of anaplastic oligodendroglial tumors and its relationship: a single institutional review of 77 patients from China.Neuro Oncol. 2012 Jan;14(1):109-16. doi: 10.1093/neuonc/nor185. Epub 2011 Oct 27. Neuro Oncol. 2012. PMID: 22039037 Free PMC article.
-
[Anaplastic glioma. Neuropathology, molecular diagnostics and current study concepts].Nervenarzt. 2010 Aug;81(8):928-30, 932-5. doi: 10.1007/s00115-010-2956-1. Nervenarzt. 2010. PMID: 20635074 Review. German.
-
O6-methylguanine DNA methyltransferase gene promoter methylation status in gliomas and its correlation with other molecular alterations: first Indian report with review of challenges for use in customized treatment.Neurosurgery. 2010 Dec;67(6):1681-91. doi: 10.1227/NEU.0b013e3181f743f5. Neurosurgery. 2010. PMID: 21107199 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

