Combined Physiotherapy and Cognitive Behavioral Therapy for Functional Movement Disorders: A Randomized Clinical Trial
- PMID: 39102249
- PMCID: PMC11385055
- DOI: 10.1001/jamaneurol.2024.2393
Combined Physiotherapy and Cognitive Behavioral Therapy for Functional Movement Disorders: A Randomized Clinical Trial
Abstract
Importance: Functional movement disorders (FMDs) are frequent and disabling neurological disorders with a substantial socioeconomic impact. Few randomized studies have analyzed the effectiveness of combined physiotherapy and psychotherapy in patients' quality of life.
Objective: To assess the efficacy of multidisciplinary treatment (physiotherapy plus cognitive behavioral therapy) in FMDs.
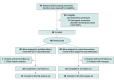
Design, setting, and participants: This was a parallel, rater-blinded, single-center, randomized clinical trial. Recruitment took place from June 2022 to April 2023, and follow-up visits were performed at months 3 and 5, concluding in October 2023. Participants were recruited from a national referral center for movement disorders: the Movement Disorders Unit from the Hospital Universitario Virgen Rocio in Seville, Spain. Patients had to be 18 years or older with a confirmed FMD diagnosis and capable of giving consent to participate. Patients who did not meet eligibility criteria or refused to participate were excluded. Any uncontrolled psychiatric disorder was considered an exclusion criterion.
Interventions: Patients were randomly assigned, in a ratio of 1:1 to multidisciplinary treatment (physiotherapy plus cognitive behavioral therapy), or a control intervention (psychological support intervention).
Main outcomes and measures: Primary outcomes: between-group differences in changes from baseline to month 3 and month 5 in patients' quality of life (EQ-5D-5L score: EQ Index and EQ visual analog scale [EQ VAS]; and 36-Item Short-Form Survey Physical Component Summary [SF-36 PCS] and SF-36 Mental Component Summary [MCS]). Linear mixed models were applied, controlling by baseline severity and applying Bonferroni correction.
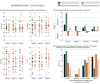
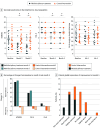
Results: Of 70 patients screened with an FMD, 40 were enrolled (mean [SD] age, 43.5 [12.8] years; age range, 18-66 years; 32 female [80%]; mean [SD] age at FMD onset, 38.4 [12.1] years), and 38 completed all the follow-up visits and were included in the analysis for primary outcomes. Multidisciplinary treatment improved SF-36 PCS with a mean between-group difference at 3 months of 4.23 points (95% CI, -0.9 to 9.4 points; P = .11) and a significant mean between-group difference at 5 months of 5.62 points (95% CI, 2.3-8.9 points; P < .001), after multiple-comparisons adjustment. There were no significant differences in other quality-of-life outcomes such as SF-36 MCS (mean between-group difference at 3 and 5 months: 0.72 points; 95% CI, -5.5 to 7.0 points; P = .82 and 0.69 points; 95% CI, 2.3-8.9 points; P = .83, respectively), EQ VAS (9.34 points; 95% CI, -0.6 to 19.3 points; P = .07 and 13.7 points; 95% CI, -1.7 to 29.0 points; P = .09, respectively) and EQ Index (0.001 point; 95% CI, -0.1 to 0.1 point; P = .98 and 0.08 points; 95% CI, 0-0.2 points; P = .13, respectively). At months 3 and 5, 42% and 47% of patients, respectively, in the multidisciplinary group reported improved health using the EQ-5D system, compared with 26% and 16% of patients, respectively, in the control group.
Conclusions and relevance: Results show that multidisciplinary treatment (physiotherapy plus cognitive behavioral therapy) effectively improves FMD symptoms and physical aspects of patients' quality of life. Further studies must be performed to evaluate the potential cost-effectiveness of this approach in FMD.
Trial registration: ClinicalTrials.gov Identifier: NCT05634486.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

