MRI and Clinical Variables for Prediction of Outcomes After Pediatric Severe Traumatic Brain Injury
- PMID: 39102267
- PMCID: PMC11301548
- DOI: 10.1001/jamanetworkopen.2024.25765
MRI and Clinical Variables for Prediction of Outcomes After Pediatric Severe Traumatic Brain Injury
Abstract
Importance: Traumatic brain injury (TBI) is a leading cause of death and disability in children, and predicting functional outcome after TBI is challenging. Magnetic resonance imaging (MRI) is frequently conducted after severe TBI; however, the predictive value of MRI remains uncertain.
Objectives: To identify early MRI measures that predict long-term outcome after severe TBI in children and to assess the added predictive value of MRI measures over well-validated clinical predictors.
Design, setting, and participants: This preplanned prognostic study used data from the Approaches and Decisions in Acute Pediatric TBI (ADAPT) prospective observational comparative effectiveness study. The ADAPT study enrolled 1000 consecutive children (aged <18 years) with severe TBI between February 1, 2014, and September 30, 2017. Participants had a Glasgow Coma Scale (GCS) score of 8 or less and received intracranial pressure monitoring. Magnetic resonance imaging scans performed as part of standard clinical care within 30 days of injury were collected at 24 participating sites in the US, UK, and Australia. Summary imaging measures were correlated with the Glasgow Outcome Scale-Extended for Pediatrics (GOSE-Peds), and the predictive value of MRI measures was compared with the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) core clinical predictors. Data collection, image analysis, and data analyses were completed in July 2023.
Exposures: Pediatric severe TBI with an MRI scan performed as part of clinical care.
Main outcomes and measures: All measures were selected a priori. Magnetic resonance imaging measures included contusion, ischemia, diffuse axonal injury, intracerebral hemorrhage, and brainstem injury. Clinical predictors included the IMPACT core measures (GCS motor score and pupil reactivity). All models adjusted for age and sex. Outcome measures included the GOSE-Peds score obtained at 3, 6, and 12 months after injury.
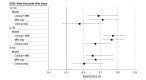
Results: This study included 233 children with severe TBI who were enrolled at participating sites and had an MRI scan and preselected clinical predictors available. Their median age was 6.9 (IQR, 3.0-13.3) years, and more than half of participants (134 [57.5%]) were male. In a multivariable model including MRI measures and IMPACT core clinical variables, contusion volume (odds ratio [OR], 1.13; 95% CI, 1.02-1.26), brain ischemia (OR, 2.11; 95% CI, 1.58-2.81), brainstem lesions (OR, 5.40; 95% CI, 1.90-15.35), and pupil reactivity were each independently associated with GOSE-Peds score. Adding MRI measures to the IMPACT clinical predictors significantly improved model fit and discrimination between favorable and unfavorable outcomes compared with IMPACT predictors alone (area under the receiver operating characteristic curve, 0.77; 95% CI, 0.72-0.85 vs 0.67; 95% CI, 0.61-0.76 for GOSE-Peds score >3 at 6 months after injury).
Conclusions and relevance: In this prognostic study of children with severe TBI, the addition of MRI measures significantly improved outcome prediction over well-established and validated clinical predictors. Magnetic resonance imaging should be considered in children with severe TBI to inform prognosis and may also promote stratification of patients in future clinical trials.
Conflict of interest statement
Figures
Comment in
-
Beyond the AJR: Can MRI Predict Outcomes in Children With Severe Traumatic Brain Injury?AJR Am J Roentgenol. 2025 Aug;225(2):e2432312. doi: 10.2214/AJR.24.32312. Epub 2024 Dec 11. AJR Am J Roentgenol. 2025. PMID: 39660832 No abstract available.
References
-
- Zaloshnja E, Miller T, Langlois JA, Selassie AW. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil. 2008;23(6):394-400. doi:10.1097/01.HTR.0000341435.52004.ac - DOI - PubMed
-
- Emami P, Czorlich P, Fritzsche FS, et al. . Impact of Glasgow Coma Scale score and pupil parameters on mortality rate and outcome in pediatric and adult severe traumatic brain injury: a retrospective, multicenter cohort study. J Neurosurg. 2017;126(3):760-767. doi:10.3171/2016.1.JNS152385 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

