A novel kinetic model to demonstrate the independent effects of ATP and ADP/Pi concentrations on sarcomere function
- PMID: 39102392
- PMCID: PMC11326600
- DOI: 10.1371/journal.pcbi.1012321
A novel kinetic model to demonstrate the independent effects of ATP and ADP/Pi concentrations on sarcomere function
Abstract
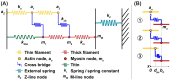
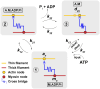
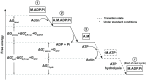
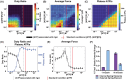
Understanding muscle contraction mechanisms is a standing challenge, and one of the approaches has been to create models of the sarcomere-the basic contractile unit of striated muscle. While these models have been successful in elucidating many aspects of muscle contraction, they fall short in explaining the energetics of functional phenomena, such as rigor, and in particular, their dependence on the concentrations of the biomolecules involved in the cross-bridge cycle. Our hypothesis posits that the stochastic time delay between ATP adsorption and ADP/Pi release in the cross-bridge cycle necessitates a modeling approach where the rates of these two reaction steps are controlled by two independent parts of the total free energy change of the hydrolysis reaction. To test this hypothesis, we built a two-filament, stochastic-mechanical half-sarcomere model that separates the energetic roles of ATP and ADP/Pi in the cross-bridge cycle's free energy landscape. Our results clearly demonstrate that there is a nontrivial dependence of the cross-bridge cycle's kinetics on the independent concentrations of ATP, ADP, and Pi. The simplicity of the proposed model allows for analytical solutions of the more basic systems, which provide novel insight into the dominant mechanisms driving some of the experimentally observed contractile phenomena.
Copyright: © 2024 Schmidt et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Ingwall JS, Weiss RG. Is the Failing Heart Energy Starved? Circulation Research. 2004;95(2):135–145. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

