Impact of waning immunity against SARS-CoV-2 severity exacerbated by vaccine hesitancy
- PMID: 39102402
- PMCID: PMC11299835
- DOI: 10.1371/journal.pcbi.1012211
Impact of waning immunity against SARS-CoV-2 severity exacerbated by vaccine hesitancy
Abstract
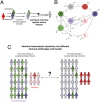
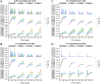
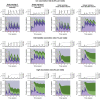
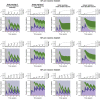
The SARS-CoV-2 pandemic has generated a considerable number of infections and associated morbidity and mortality across the world. Recovery from these infections, combined with the onset of large-scale vaccination, have led to rapidly-changing population-level immunological landscapes. In turn, these complexities have highlighted a number of important unknowns related to the breadth and strength of immunity following recovery or vaccination. Using simple mathematical models, we investigate the medium-term impacts of waning immunity against severe disease on immuno-epidemiological dynamics. We find that uncertainties in the duration of severity-blocking immunity (imparted by either infection or vaccination) can lead to a large range of medium-term population-level outcomes (i.e. infection characteristics and immune landscapes). Furthermore, we show that epidemiological dynamics are sensitive to the strength and duration of underlying host immune responses; this implies that determining infection levels from hospitalizations requires accurate estimates of these immune parameters. More durable vaccines both reduce these uncertainties and alleviate the burden of SARS-CoV-2 in pessimistic outcomes. However, heterogeneity in vaccine uptake drastically changes immune landscapes toward larger fractions of individuals with waned severity-blocking immunity. In particular, if hesitancy is substantial, more robust vaccines have almost no effects on population-level immuno-epidemiology, even if vaccination rates are compensatorily high among vaccine-adopters. This pessimistic scenario for vaccination heterogeneity arises because those few individuals that are vaccine-adopters are so readily re-vaccinated that the duration of vaccinal immunity has no appreciable consequences on their immune status. Furthermore, we find that this effect is heightened if vaccine-hesitants have increased transmissibility (e.g. due to riskier behavior). Overall, our results illustrate the necessity to characterize both transmission-blocking and severity-blocking immune time scales. Our findings also underline the importance of developing robust next-generation vaccines with equitable mass vaccine deployment.
Copyright: © 2024 Saad-Roy et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press; 1991.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

