Toward real-time reporting of cancer incidence: methodology, pilot study, and SEER Program implementation
- PMID: 39102887
- PMCID: PMC12104527
- DOI: 10.1093/jncimonographs/lgae024
Toward real-time reporting of cancer incidence: methodology, pilot study, and SEER Program implementation
Abstract
Background: A lag time between cancer case diagnosis and incidence reporting impedes the ability to monitor the impact of recent events on cancer incidence. Currently, the data submission standard is 22 months after a diagnosis year ends, and the reporting standard is 27.5 months after a diagnosis year ends. This paper presents the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program's efforts to minimize the lag and achieve "real-time" reporting, operationalized as submission within 2 months from the end of a diagnosis year.
Methods: Technology for rapidly creating a consolidated tumor case (CTC) from electronic pathology (e-path) reports is described. Statistical methods are extended to adjust for biases in incidence rates due to reporting delays for the most recent diagnosis years.
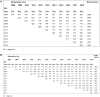
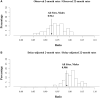
Results: A registry pilot study demonstrated that real-time submissions can approximate rates obtained from 22-month submissions after adjusting for reporting delays. A plan to be implemented across the SEER Program rapidly ascertains unstructured e-path reports and uses machine learning algorithms to translate the reports into the core data items that comprise a CTC for incidence reporting. Across the program, cases were submitted 2 months after the end of the calendar year. Registries with the most promising baseline values and a willingness to modify registry operations have joined a program to become certified as real-time reporting.
Conclusion: Advances in electronic reporting, natural language processing, registry operations, and statistical methodology, energized by the SEER Program's mobilization and coordination of these efforts, will make real-time reporting an achievable goal.
Published by Oxford University Press 2024.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

