Reporting tumor genomic test results to SEER registries via linkages
- PMID: 39102888
- PMCID: PMC11300019
- DOI: 10.1093/jncimonographs/lgae013
Reporting tumor genomic test results to SEER registries via linkages
Abstract
Background: Precision medicine has become a mainstay of cancer care in recent years. The National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program has been an authoritative source of cancer statistics and data since 1973. However, tumor genomic information has not been adequately captured in the cancer surveillance data, which impedes population-based research on molecular subtypes. To address this, the SEER Program has developed and implemented a centralized process to link SEER registries' tumor cases with genomic test results that are provided by molecular laboratories to the registries.
Methods: Data linkages were carried out following operating procedures for centralized linkages established by the SEER Program. The linkages used Match*Pro, a probabilistic linkage software, and were facilitated by the registries' trusted third party (an honest broker). The SEER registries provide to NCI limited datasets that undergo preliminary evaluation prior to their release to the research community.
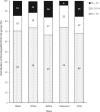
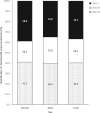
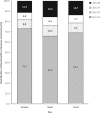
Results: Recently conducted genomic linkages included OncotypeDX Breast Recurrence Score, OncotypeDX Breast Ductal Carcinoma in Situ, OncotypeDX Genomic Prostate Score, Decipher Prostate Genomic Classifier, DecisionDX Uveal Melanoma, DecisionDX Preferentially Expressed Antigen in Melanoma, DecisionDX Melanoma, and germline tests results in Georgia and California SEER registries.
Conclusions: The linkages of cancer cases from SEER registries with genomic test results obtained from molecular laboratories offer an effective approach for data collection in cancer surveillance. By providing de-identified data to the research community, the NCI's SEER Program enables scientists to investigate numerous research inquiries.
© The Author(s) 2024. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Yadav S, Couch FJ. Germline genetic testing for breast cancer risk: the past, present, and future. Am Soc Clin Oncol Educ Book. 2019;39:61-74. - PubMed
-
- Roy-Chowdhuri S. Molecular pathology of lung cancer. Surg Pathol Clin 2021;14(3):369-377. - PubMed
-
- Beech C, Hechtman JF. Molecular approach to colorectal carcinoma: current evidence and clinical application. Surg Pathol Clin 2021;14(3):429-441. - PubMed
MeSH terms
Grants and funding
- HHSN261201800009C/CA/NCI NIH HHS/United States
- HHSN261201800005C/CA/NCI NIH HHS/United States
- 75N96021D00006/ES/NIEHS NIH HHS/United States
- HHSN261201800005I/New York State Cancer Registry
- 75N97021D00006/LM/NLM NIH HHS/United States
- HHSN261201800041C/CA/NCI NIH HHS/United States
- HHSN261201800003I/Surveillance, Epidemiology and End Results
- HHSN261201300009C/CA/NCI NIH HHS/United States
- 1NU58DP007140/CC/CDC HHS/United States
- HHSN261201800012I_HHSN26100001/Surveillance, Epidemiology and End Results Program
- University of California
- HHSN261201800012C/CA/NCI NIH HHS/United States
- HHSN261201800002I/HH/HHS/United States
- 75N92021D00009/HL/NHLBI NIH HHS/United States
- HHSN261201800001C/CA/NCI NIH HHS/United States
- HHSN261201800007I/chool of Public Health Louisiana State University Health New Orleans
- 6NU58DP006352-05-01/CC/CDC HHS/United States
- Cancer Data Registry of Idaho
- 75N98021D00006/OD/NIH HHS/United States
- HHSN261201800032C/CA/NCI NIH HHS/United States
- Emory University
- HHSN261201800004C/CA/NCI NIH HHS/United States
- Keck School of Medicine
- HHSN261201000041C/CA/NCI NIH HHS/United States
- 75N91021D00011/CA/NCI NIH HHS/United States
- 75N95021D00011/DA/NIDA NIH HHS/United States
- HHSN2612018000041/Division of Public Health Sciences Fred Hutchinson Cancer Center
- HHSN261201800013C/CA/NCI NIH HHS/United States
- HHSN261201800014C/CA/NCI NIH HHS/United States
- HHSN261201800016C/CA/NCI NIH HHS/United States
- 75N99021D00009/OF/ORFDO NIH HHS/United States
- Texas Cancer Registry
- 75N92021D00011/HL/NHLBI NIH HHS/United States
- HHSN261201300009I/CA/NCI NIH HHS/United States
- HHSN261201800016I/CA/NCI NIH HHS/United States
- 1NU58DP006270/CC/CDC HHS/United States
- 75N90021D00009/CL/CLC NIH HHS/United States
- Department of Population and Public Health Sciences
- 75N92021D00006/HL/NHLBI NIH HHS/United States
- HHSN261201800014I/CA/NCI NIH HHS/United States
- HHSN261201000041I/CA/NCI NIH HHS/United States
- HHSN261201800032I/CA/NCI NIH HHS/United States
- 75N91021D00009/New Jersey State Cancer Registry
- 75N91021D00006/Illinois State Cancer Registry
- HHSN261201800012I/CA/NCI NIH HHS/United States
- 75N96021D00009/ES/NIEHS NIH HHS/United States
- HHSN261201800002C/CA/NCI NIH HHS/United States
- HHSN261201800013I/CA/NCI NIH HHS/United States
- HHSN261201800006I/CA/NCI NIH HHS/United States
- HHSN261201800009I/CA/NCI NIH HHS/United States
- HHSN261201800002B/CA/NCI NIH HHS/United States
- HHSN261201800007C/CA/NCI NIH HHS/United States
- HHSN261201800003C/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

