GDF11 protects against mitochondrial-dysfunction-dependent NLRP3 inflammasome activation to attenuate osteoarthritis
- PMID: 39103049
- PMCID: PMC12225960
- DOI: 10.1016/j.jare.2024.08.001
GDF11 protects against mitochondrial-dysfunction-dependent NLRP3 inflammasome activation to attenuate osteoarthritis
Abstract
Introduction: Osteoarthritis (OA) is a highly prevalent degenerative disease worldwide, and tumor necrosis factor (TNF-α) is closely associated with its development. Growth differentiation factor 11 (GDF11) has demonstrated anti-injury and anti-aging abilities in certain tissues; however, its regulatory role in OA remains unclear and requires further investigation.
Objectives: To identify whether GDF11 can attenuate osteoarthritis. To exploring the the potential mechanism of GDF11 in alleviating osteoarthritis.
Methods: In this study, we cultured and stimulated mouse primary chondrocytes with or without TNF-α, analyzing the resulting damage phenotype through microarray analysis. Additionally, we employed GDF11 conditional knockout mice OA model to examine the relationship between GDF11 and OA. To investigate the target of GDF11's function, we utilized NLRP3 knockout mice and its inhibitor to verify the potential involvement of the NLRP3 inflammasome.
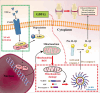
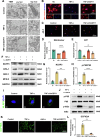
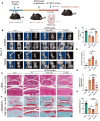
Results: Our in vitro experiments demonstrated that endogenous overexpression of GDF11 significantly inhibited TNF-α-induced cartilage matrix degradation and inflammatory expression in chondrocytes. Furthermore, loss of GDF11 led to NLRP3 inflammasome activation, inflammation, and metabolic dysfunction. In an in vivo surgically induced mouse model, intraarticular administration of recombinant human GDF11 alleviated OA pathogenesis, whereas GDF11 conditional knockout reversed this effect. Additionally, findings from the NLRP3-knockout DMM mouse model revealed that GDF11 exerted its protective effect by inhibiting NLRP3.
Conclusion: These findings demonstrate the ability of GDF11 to suppress TNF-α-induced inflammation and cartilage degeneration by preventing mitochondrial dysfunction and inhibiting NLRP3 inflammasome activation, suggesting its potential as a promising therapeutic drug for osteoarthritis.
Keywords: GDF11; NLRP3 inflammasome; Osteoarthritis; TNF-α.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

