Multiplexed volumetric CLEM enabled by scFvs provides insights into the cytology of cerebellar cortex
- PMID: 39103318
- PMCID: PMC11300613
- DOI: 10.1038/s41467-024-50411-z
Multiplexed volumetric CLEM enabled by scFvs provides insights into the cytology of cerebellar cortex
Abstract
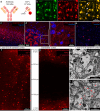
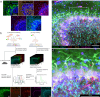
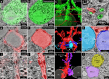
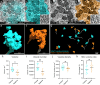
Mapping neuronal networks is a central focus in neuroscience. While volume electron microscopy (vEM) can reveal the fine structure of neuronal networks (connectomics), it does not provide molecular information to identify cell types or functions. We developed an approach that uses fluorescent single-chain variable fragments (scFvs) to perform multiplexed detergent-free immunolabeling and volumetric-correlated-light-and-electron-microscopy on the same sample. We generated eight fluorescent scFvs targeting brain markers. Six fluorescent probes were imaged in the cerebellum of a female mouse, using confocal microscopy with spectral unmixing, followed by vEM of the same sample. The results provide excellent ultrastructure superimposed with multiple fluorescence channels. Using this approach, we documented a poorly described cell type, two types of mossy fiber terminals, and the subcellular localization of one type of ion channel. Because scFvs can be derived from existing monoclonal antibodies, hundreds of such probes can be generated to enable molecular overlays for connectomic studies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Update of
-
Multiplexed volumetric CLEM enabled by antibody derivatives provides new insights into the cytology of the mouse cerebellar cortex.bioRxiv [Preprint]. 2023 Jul 7:2023.05.20.540091. doi: 10.1101/2023.05.20.540091. bioRxiv. 2023. Update in: Nat Commun. 2024 Aug 5;15(1):6648. doi: 10.1038/s41467-024-50411-z. PMID: 37292964 Free PMC article. Updated. Preprint.
-
Multiplexed volumetric CLEM enabled by antibody derivatives provides new insights into the cytology of the mouse cerebellar cortex.Res Sq [Preprint]. 2023 Jul 6:rs.3.rs-3121892. doi: 10.21203/rs.3.rs-3121892/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Aug 5;15(1):6648. doi: 10.1038/s41467-024-50411-z. PMID: 37461609 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

