Induction of osteoblast apoptosis stimulates macrophage efferocytosis and paradoxical bone formation
- PMID: 39103355
- PMCID: PMC11300627
- DOI: 10.1038/s41413-024-00341-9
Induction of osteoblast apoptosis stimulates macrophage efferocytosis and paradoxical bone formation
Abstract
Apoptosis is crucial for tissue homeostasis and organ development. In bone, apoptosis is recognized to be a main fate of osteoblasts, yet the relevance of this process remains underexplored. Using our murine model with inducible Caspase 9, the enzyme that initiates intrinsic apoptosis, we triggered apoptosis in a proportion of mature osteocalcin (OCN+) osteoblasts and investigated the impact on postnatal bone development. Osteoblast apoptosis stimulated efferocytosis by osteal macrophages. A five-week stimulation of OCN+ osteoblast apoptosis in 3-week-old male and female mice significantly enhanced vertebral bone formation while increasing osteoblast precursors. A similar treatment regimen to stimulate osterix+ cell apoptosis had no impact on bone volume or density. The vertebral bone accrual following stimulation of OCN+ osteoblast apoptosis did not translate in improved mechanical strength due to disruption of the lacunocanalicular network. The observed bone phenotype was not influenced by changes in osteoclasts but was associated with stimulation of macrophage efferocytosis and vasculature formation. Phenotyping of efferocytic macrophages revealed a unique transcriptomic signature and expression of factors including VEGFA. To examine whether macrophages participated in the osteoblast precursor increase following osteoblast apoptosis, macrophage depletion models were employed. Depletion of macrophages via clodronate-liposomes and the CD169-diphtheria toxin receptor mouse model resulted in marked reduction in leptin receptor+ and osterix+ osteoblast precursors. Collectively, this work demonstrates the significance of osteoblast turnover via apoptosis and efferocytosis in postnatal bone formation. Importantly, it exposes the potential of targeting this mechanism to promote bone anabolism in the clinical setting.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

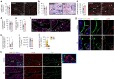



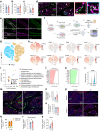

References
MeSH terms
Grants and funding
- CA093900/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P30 CA046592/CA/NCI NIH HHS/United States
- APP1143802/Department of Health | National Health and Medical Research Council (NHMRC)
- R01 DK053904/DK/NIDDK NIH HHS/United States
- P30 AR069620/AR/NIAMS NIH HHS/United States
- AR077539/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- P01 CA093900/CA/NCI NIH HHS/United States
- R56 AR077539/AR/NIAMS NIH HHS/United States
- R01 DE022327/DE/NIDCR NIH HHS/United States
- AR069620/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R56 DK053904/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

