Solution-derived Ge-Sb-Se-Te phase-change chalcogenide films
- PMID: 39103371
- PMCID: PMC11300797
- DOI: 10.1038/s41598-024-69045-8
Solution-derived Ge-Sb-Se-Te phase-change chalcogenide films
Abstract
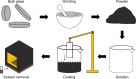
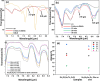
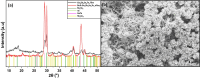
Ge-Sb-Se-Te chalcogenides, namely Se-substituted Ge-Sb-Te, have been developed as an alternative optical phase change material (PCM) with a high figure-of-merit. A need for the integration of such new PCMs onto a variety of photonic platforms has necessitated the development of fabrication processes compatible with diverse material compositions as well as substrates of varying material types, shapes, and sizes. This study explores the application of chemical solution deposition as a method capable of creating conformally coated layers and delves into the resulting modifications in the structural and optical properties of Ge-Sb-Se-Te PCMs. Specifically, we detail the solution-based deposition of Ge-Sb-Se-Te layers and present a comparative analysis with those deposited via thermal evaporation. We also discuss our ongoing endeavor to improve available choice of processing-material combinations and how to realize solution-derived high figure-of-merit optical PCM layers, which will enable a new era for the development of reconfigurable photonic devices.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Wuttig, M., Bhaskaran, H. & Taubner, T. Phase-change materials for non-volatile photonic applications. Nat. Photon.11, 465 (2017). 10.1038/nphoton.2017.126 - DOI
-
- Friedrich, I., Weidenhof, V., Njoroge, W., Franz, P. & Wuttig, M. Structural transformations of Ge2Sb2Te5 films studied by electrical resistance measurements. J. Appl. Phys.87, 4130 (2000). 10.1063/1.373041 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

