Insulin enhances acid-sensing ion channel currents in rat primary sensory neurons
- PMID: 39103432
- PMCID: PMC11300854
- DOI: 10.1038/s41598-024-69139-3
Insulin enhances acid-sensing ion channel currents in rat primary sensory neurons
Abstract
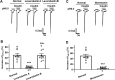
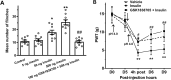
Insulin has been shown to modulate neuronal processes through insulin receptors. The ion channels located on neurons may be important targets for insulin/insulin receptor signaling. Both insulin receptors and acid-sensing ion channels (ASICs) are expressed in dorsal root ganglia (DRG) neurons. However, it is still unclear whether there is an interaction between them. Therefore, the purpose of this investigation was to determine the effects of insulin on the functional activity of ASICs. A 5 min application of insulin rapidly enhanced acid-evoked ASIC currents in rat DRG neurons in a concentration-dependent manner. Insulin shifted the concentration-response plot for ASIC currents upward, with an increase of 46.2 ± 7.6% in the maximal current response. The insulin-induced increase in ASIC currents was eliminated by the insulin receptor antagonist GSK1838705, the tyrosine kinase inhibitor lavendustin A, and the phosphatidylinositol-3 kinase antagonist wortmannin. Moreover, insulin increased the number of acid-triggered action potentials by activating insulin receptors. Finally, local administration of insulin exacerbated the spontaneous nociceptive behaviors induced by intraplantar acid injection and the mechanical hyperalgesia induced by intramuscular acid injections through peripheral insulin receptors. These results suggested that insulin/insulin receptor signaling enhanced the functional activity of ASICs via tyrosine kinase and phosphatidylinositol-3 kinase pathways. Our findings revealed that ASICs were targets in primary sensory neurons for insulin receptor signaling, which may underlie insulin modulation of pain.
Keywords: Acid-sensing ion channel; Current; Dorsal root ganglion neuron; Insulin; Nociceptive behavior.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



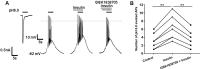
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

