PQBP3 prevents senescence by suppressing PSME3-mediated proteasomal Lamin B1 degradation
- PMID: 39103492
- PMCID: PMC11405525
- DOI: 10.1038/s44318-024-00192-4
PQBP3 prevents senescence by suppressing PSME3-mediated proteasomal Lamin B1 degradation
Abstract
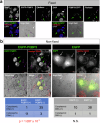
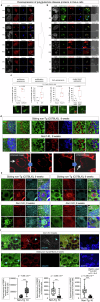
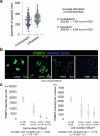
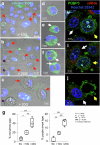
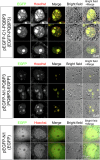
Senescence of nondividing neurons remains an immature concept, with especially the regulatory molecular mechanisms of senescence-like phenotypes and the role of proteins associated with neurodegenerative diseases in triggering neuronal senescence remaining poorly explored. In this study, we reveal that the nucleolar polyglutamine binding protein 3 (PQBP3; also termed NOL7), which has been linked to polyQ neurodegenerative diseases, regulates senescence as a gatekeeper of cytoplasmic DNA leakage. PQBP3 directly binds PSME3 (proteasome activator complex subunit 3), a subunit of the 11S proteasome regulator complex, decreasing PSME3 interaction with Lamin B1 and thereby preventing Lamin B1 degradation and senescence. Depletion of endogenous PQBP3 causes nuclear membrane instability and release of genomic DNA from the nucleus to the cytosol. Among multiple tested polyQ proteins, ataxin-1 (ATXN1) partially sequesters PQBP3 to inclusion bodies, reducing nucleolar PQBP3 levels. Consistently, knock-in mice expressing mutant Atxn1 exhibit decreased nuclear PQBP3 and a senescence phenotype in Purkinje cells of the cerebellum. Collectively, these results suggest homologous roles of the nucleolar protein PQBP3 in cellular senescence and neurodegeneration.
Keywords: Lamin B1; Neurodegeneration; Nuclear Membrane Instability; PQBP3; Senescence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
PQBP3/NOL7 is an intrinsically disordered protein.Biochem Biophys Res Commun. 2024 Dec 3;736:150453. doi: 10.1016/j.bbrc.2024.150453. Epub 2024 Jul 31. Biochem Biophys Res Commun. 2024. PMID: 39126896
-
E3 ubiquitin ligase RNF123 targets lamin B1 and lamin-binding proteins.FEBS J. 2018 Jun;285(12):2243-2262. doi: 10.1111/febs.14477. Epub 2018 May 3. FEBS J. 2018. PMID: 29676528
-
E3 ubiquitin ligase HECW2 targets PCNA and lamin B1.Biochim Biophys Acta Mol Cell Res. 2018 Aug;1865(8):1088-1104. doi: 10.1016/j.bbamcr.2018.05.008. Epub 2018 May 17. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29753763
-
Progress in pathogenesis studies of spinocerebellar ataxia type 1.Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1079-81. doi: 10.1098/rstb.1999.0462. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434309 Free PMC article. Review.
-
PA200-Mediated Proteasomal Protein Degradation and Regulation of Cellular Senescence.Int J Mol Sci. 2024 May 22;25(11):5637. doi: 10.3390/ijms25115637. Int J Mol Sci. 2024. PMID: 38891826 Free PMC article. Review.
References
-
- Barclay SS, Tamura T, Ito H, Fujita K, Tagawa K, Shimamura T, Katsuta A, Shiwaku H, Sone M, Imoto S, Miyano S, Okazawa H (2014) Systems biology analysis of Drosophila in vivo screen data elucidates core networks for DNA damage repair in SCA1. Hum Mol Genet 23:1345–1364 10.1093/hmg/ddt524 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 22110001/ 22110002/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
- 16H02655/MEXT | Japan Society for the Promotion of Science (JSPS)
- 19H01042/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22H00464/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP24gm1910001/Japan Agency for Medical Research and Development (AMED)
LinkOut - more resources
Full Text Sources
Research Materials

