Development of 3D-iNET ORION: a novel, pre-clinical, three-dimensional in vitro cell model for modeling human metastatic neuroendocrine tumor of the pancreas
- PMID: 39103560
- PMCID: PMC11341600
- DOI: 10.1007/s13577-024-01113-7
Development of 3D-iNET ORION: a novel, pre-clinical, three-dimensional in vitro cell model for modeling human metastatic neuroendocrine tumor of the pancreas
Abstract
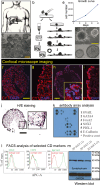
Neuroendocrine tumors (NETs) of the pancreas are rare neoplasms that present complex challenges to diagnosis and treatment due to their indolent course. The incidence of pancreatic neuroendocrine tumors has increased significantly over the past two decades. A limited number of pancreatic neuroendocrine cell lines are currently available for the research. Here, we present 3D-iNET ORION, a novel 3-dimensional (spheroid) cell line, isolated from human pancreatic neuroendocrine tumor liver metastasis. Three-dimensionally grown (3D) cancer cell lines have gained interest over the past years as 3D cancer cell lines better recapitulate the in vivo structure of tumors, and are more suitable for in vitro and in vivo experiments. 3D-iNET ORION cancer cell line showed high potential to form tumorspheres when embedded in Matrigel matrix and expresses synaptophysin and EpCAM. Electron microscopy analysis of cancer cell line proved the presence of dense neurosecretory granules. When xenografted into athymic mice, 3D-iNET ORION cells produce slow-growing tumors, positive for chromogranin and synaptophysin. Human Core Exome Panel Analysis has shown that 3DiNET ORION cell line retains the genetic aberration profile detected in the original tumor. In conclusion, our newly developed neuroendocrine cancer cell line can be considered as a new research tool for in vitro and in vivo experiments.
Keywords: 3D cancer cell lines; Neuroendocrine tumor; Pancreas.
© 2024. The Author(s).
Conflict of interest statement
All authors declare no conflict of interest.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

