Progesterone boosts abiraterone-driven target and NK cell therapies against glioblastoma
- PMID: 39103871
- PMCID: PMC11302026
- DOI: 10.1186/s13046-024-03144-2
Progesterone boosts abiraterone-driven target and NK cell therapies against glioblastoma
Abstract
Introduction: Glioblastoma (GBM) poses a significant challenge in oncology, with median survival times barely extending beyond a year due to resistance to standard therapies like temozolomide (TMZ). This study introduces a novel therapeutic strategy combining progesterone (Prog) and abiraterone (Abi) aimed at enhancing GBM treatment efficacy by modulating the tumor microenvironment and augmenting NK cell-mediated immunity.
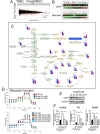
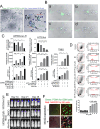
Methods: We employed in vitro and in vivo GBM models to assess the effects of Prog and Abi on cell viability, proliferation, apoptosis, and the immune microenvironment. Techniques included cell viability assays, Glo-caspase 3/7 apoptosis assays, RNA-seq and qPCR for gene expression, Seahorse analysis for mitochondrial function, HPLC-MS for metabolomics analysis, and immune analysis by flow cytometry to quantify NK cell infiltration.
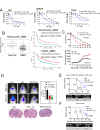
Results: Prog significantly reduced the IC50 of Abi in TMZ-resistant GBM cell, suggesting the enhanced cytotoxicity. Treatment induced greater apoptosis than either agent alone, suppressed tumor growth, and prolonged survival in mouse models. Notably, there was an increase in CD3-/CD19-/CD56+/NK1.1+ NK cell infiltration in treated tumors, indicating a shift towards an anti-tumor immune microenvironment. The combination therapy also resulted in a reduction of MGMT expression and a suppression of mitochondrial respiration and glycolysis in GBM cells.
Conclusion: The combination of Prog and Abi represents a promising therapeutic approach for GBM, showing potential in suppressing tumor growth, extending survival, and modulating the immune microenvironment. These findings warrant further exploration into the clinical applicability of this strategy to improve outcomes for GBM patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

