Phenotyping COVID-19 respiratory failure in spontaneously breathing patients with AI on lung CT-scan
- PMID: 39103945
- PMCID: PMC11301830
- DOI: 10.1186/s13054-024-05046-3
Phenotyping COVID-19 respiratory failure in spontaneously breathing patients with AI on lung CT-scan
Abstract
Background: Automated analysis of lung computed tomography (CT) scans may help characterize subphenotypes of acute respiratory illness. We integrated lung CT features measured via deep learning with clinical and laboratory data in spontaneously breathing subjects to enhance the identification of COVID-19 subphenotypes.
Methods: This is a multicenter observational cohort study in spontaneously breathing patients with COVID-19 respiratory failure exposed to early lung CT within 7 days of admission. We explored lung CT images using deep learning approaches to quantitative and qualitative analyses; latent class analysis (LCA) by using clinical, laboratory and lung CT variables; regional differences between subphenotypes following 3D spatial trajectories.
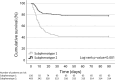
Results: Complete datasets were available in 559 patients. LCA identified two subphenotypes (subphenotype 1 and 2). As compared with subphenotype 2 (n = 403), subphenotype 1 patients (n = 156) were older, had higher inflammatory biomarkers, and were more hypoxemic. Lungs in subphenotype 1 had a higher density gravitational gradient with a greater proportion of consolidated lungs as compared with subphenotype 2. In contrast, subphenotype 2 had a higher density submantellar-hilar gradient with a greater proportion of ground glass opacities as compared with subphenotype 1. Subphenotype 1 showed higher prevalence of comorbidities associated with endothelial dysfunction and higher 90-day mortality than subphenotype 2, even after adjustment for clinically meaningful variables.
Conclusions: Integrating lung-CT data in a LCA allowed us to identify two subphenotypes of COVID-19, with different clinical trajectories. These exploratory findings suggest a role of automated imaging characterization guided by machine learning in subphenotyping patients with respiratory failure.
Trial registration: ClinicalTrials.gov Identifier: NCT04395482. Registration date: 19/05/2020.
Keywords: Artificial intelligence; COVID-19; Computed tomography; Latent class analysis; Respiratory failure; Subphenotypes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Shah FA, Meyer NJ, Angus DC, Awdish R, Azoulay É, Calfee CS, et al. A research agenda for precision medicine in sepsis and acute respiratory distress syndrome: an official American Thoracic Society Research statement. Am J Respir Crit Care Med. 2021;204(8):891–901. 10.1164/rccm.202108-1908ST - DOI - PMC - PubMed

