A pragmatic, stepped-wedge, hybrid type II trial of interoperable clinical decision support to improve venous thromboembolism prophylaxis for patients with traumatic brain injury
- PMID: 39103955
- PMCID: PMC11299263
- DOI: 10.1186/s13012-024-01386-4
A pragmatic, stepped-wedge, hybrid type II trial of interoperable clinical decision support to improve venous thromboembolism prophylaxis for patients with traumatic brain injury
Abstract
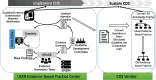
Background: Venous thromboembolism (VTE) is a preventable medical condition which has substantial impact on patient morbidity, mortality, and disability. Unfortunately, adherence to the published best practices for VTE prevention, based on patient centered outcomes research (PCOR), is highly variable across U.S. hospitals, which represents a gap between current evidence and clinical practice leading to adverse patient outcomes. This gap is especially large in the case of traumatic brain injury (TBI), where reluctance to initiate VTE prevention due to concerns for potentially increasing the rates of intracranial bleeding drives poor rates of VTE prophylaxis. This is despite research which has shown early initiation of VTE prophylaxis to be safe in TBI without increased risk of delayed neurosurgical intervention or death. Clinical decision support (CDS) is an indispensable solution to close this practice gap; however, design and implementation barriers hinder CDS adoption and successful scaling across health systems. Clinical practice guidelines (CPGs) informed by PCOR evidence can be deployed using CDS systems to improve the evidence to practice gap. In the Scaling AcceptabLE cDs (SCALED) study, we will implement a VTE prevention CPG within an interoperable CDS system and evaluate both CPG effectiveness (improved clinical outcomes) and CDS implementation.
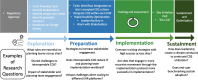
Methods: The SCALED trial is a hybrid type 2 randomized stepped wedge effectiveness-implementation trial to scale the CDS across 4 heterogeneous healthcare systems. Trial outcomes will be assessed using the RE2-AIM planning and evaluation framework. Efforts will be made to ensure implementation consistency. Nonetheless, it is expected that CDS adoption will vary across each site. To assess these differences, we will evaluate implementation processes across trial sites using the Exploration, Preparation, Implementation, and Sustainment (EPIS) implementation framework (a determinant framework) using mixed-methods. Finally, it is critical that PCOR CPGs are maintained as evidence evolves. To date, an accepted process for evidence maintenance does not exist. We will pilot a "Living Guideline" process model for the VTE prevention CDS system.
Discussion: The stepped wedge hybrid type 2 trial will provide evidence regarding the effectiveness of CDS based on the Berne-Norwood criteria for VTE prevention in patients with TBI. Additionally, it will provide evidence regarding a successful strategy to scale interoperable CDS systems across U.S. healthcare systems, advancing both the fields of implementation science and health informatics.
Trial registration: Clinicaltrials.gov - NCT05628207. Prospectively registered 11/28/2022, https://classic.
Clinicaltrials: gov/ct2/show/NCT05628207 .
Keywords: Clinical decision support; Health informatics; Implementation science; Learning health system; Mixed methods; Prophylaxis; Randomized controlled trial; Stepped wedge; Traumatic brain injury; Venous thromboembolism.
© 2024. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures
References
-
- Yorkgitis BK, Berndtson AE, Cross A, Kennedy R, Kochuba MP, Tignanelli C, Tominaga GT, Jacobs DG, Marx WH, Ashley DW, Ley EJ, Napolitano L, Costantini TW. American Association for the Surgery of Trauma/American College of Surgeons-Committee on Trauma Clinical Protocol for inpatient venous thromboembolism prophylaxis after trauma. J Trauma Acute Care Surg. 2022;92(3):597–604. 10.1097/TA.0000000000003475 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous